Production method of enteric-coated kitasamycin for feed
A guitarmycin and production method technology, applied in animal feed, animal feed, application and other directions, can solve the problems of stomach irritation, nausea, inhibition of difficult pathogenic microorganisms, etc., to prolong the release and action time, and reduce the cost of medication , the effect of reducing the frequency of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
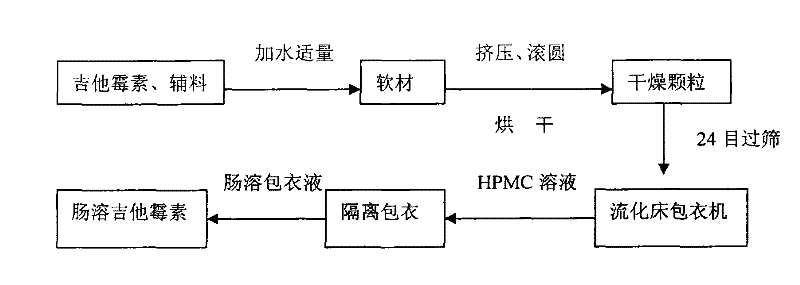
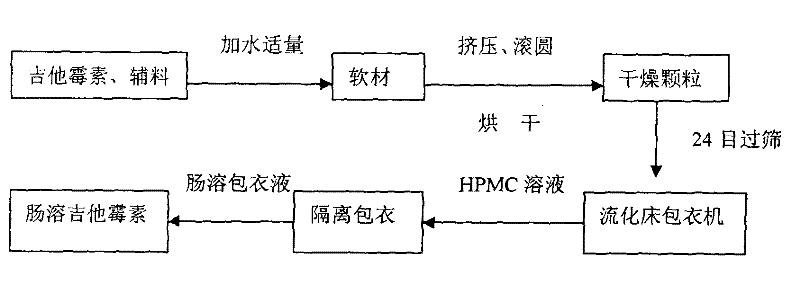
[0018] Use 35kg of kitasamycin, 55.5kg of starch, 6.5kg of dextrin, and 3kg of mannitol to mix evenly through a 60-mesh sieve, add 8% of water to make a soft material, and then extrude the sieve plate (aperture 0.8mm) to form an equivalent diameter Strips. Then enter the spheronizer to completely spheronize the particles, dry at 40°C, and take 24-mesh pellets for coating. First use 3.0% HPMC (hydroxypropyl methylcellulose) aqueous solution as a slow-release coating, and dry at 40°C for about 10 minutes. Then use 7kg acrylic resin (enteric-coating type) as the coating material for enteric coating, and all of them are coated with a fluidized bed coater. The coating process conditions are as follows: blower frequency: 27.5 Hz; jet pressure: 0.2 MPa; spray flow rate: 1 ml / min; fluidization temperature: 33°C.
Embodiment 2
[0020] Use 30kg of kitasamycin, 60kg of starch, 6.5kg of dextrin, and 3.5kg of mannitol to pass through a 60-mesh sieve and mix evenly, add 7% of water to make a soft material, and extrude the sieve plate (aperture 0.8mm) to form an equivalent diameter Strips. Then enter the spheronizer to completely spheronize the particles, dry at 40°C, and take 24-mesh pellets for coating. First use 3.0% HPMC (hydroxypropyl methylcellulose) aqueous solution as a slow-release coating, and dry at 40°C for about 10 minutes. Then use 6 kg of acrylic resin (enteric-coating type) as a coating material for enteric coating, all of which are coated with a fluidized bed coater. The coating process conditions are as follows: blower frequency: 27.5 Hz; jet pressure: 0.2 MPa; spray flow rate: 1 ml / min; fluidization temperature: 33°C.
Embodiment 3
[0022] Use 25kg of kitasamycin, 64kg of starch, 7.5kg of dextrin, and 3.5kg of mannitol to mix evenly through a 60-mesh sieve, add 6% of water to make a soft material, and extrude the sieve plate (aperture 0.8mm) to form an equivalent diameter Strips. Then enter the spheronizer to completely spheronize the particles, dry at 40°C, and take 24-mesh pellets for coating. Use 3.0% HPMC (hydroxypropyl methyl cellulose) aqueous solution as slow-release coating, and dry at 40°C for about 10 minutes. 5kg acrylic resin (enteric-coated type) was used as coating material for enteric coating, and all were coated with a fluidized bed coater. The coating process conditions are as follows: blower frequency: 27.5 Hz; jet pressure: 0.2 MPa; spray flow rate: 1 ml / min; fluidization temperature: 33°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com