Use of Bifidobacterium animalis A6 in the preparation of medicaments
An animal bifidobacteria and a technology for preparing medicines are used in pharmaceutical compositions for treating or preventing antibiotic-related diarrhea. In the field of medicine preparation, Bifidobacterium animalis A6 can solve the problem that the treatment of antibiotic-related diarrhea needs further research and the like, and achieves High medicinal and therapeutic value, increased water content, increased variety of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1 Establishment of Antibiotic-Associated Diarrhea Mouse Model
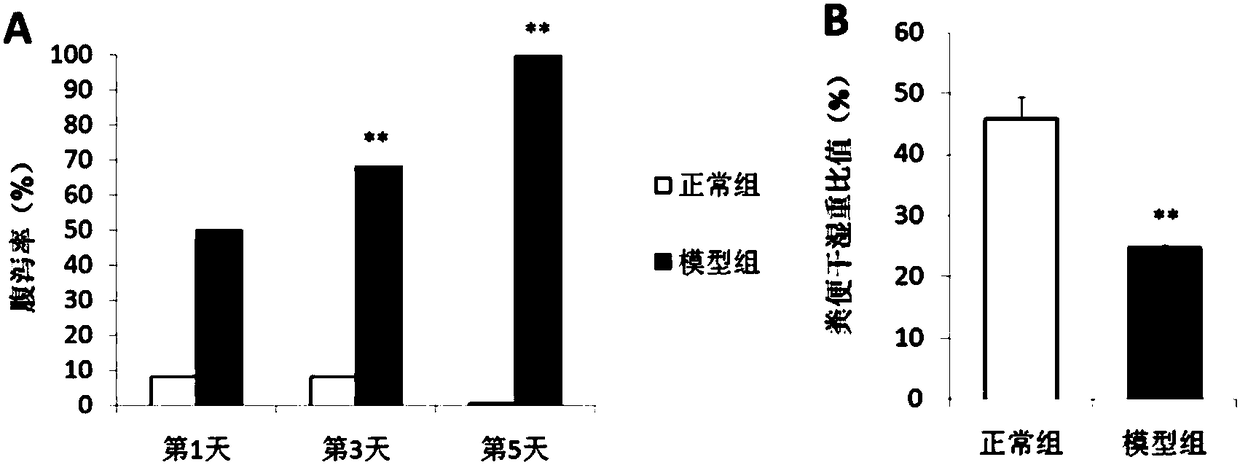
[0140] 1.1 Changes in the diarrhea rate of mice during the modeling period
[0141] Normal mice have shiny hair, normal activities, small hard particles in feces, and low water content. The mice in the model group were listless, their hair was dull, and their feces changed. Gavage ceftriaxone sodium solution, 0.2 mL of ceftriaxone sodium solution of 250 mg / mL in BALB / c mice every day, after 5 consecutive days, the mouse feces were fluffy and wet, and the stool grains became longer, and mild diarrhea gradually occurred. Reach peak after 5 days of administration, all mice all produce diarrhea (results see figure 1 A).
[0142] 1.2 Changes in the dry-wet weight ratio of mouse feces during the modeling period
[0143] After five days of administration, all the mice in the model group developed mild diarrhea. The inventor further used the ratio of dry and wet weight of feces to reflect the degree of ...
Embodiment 2
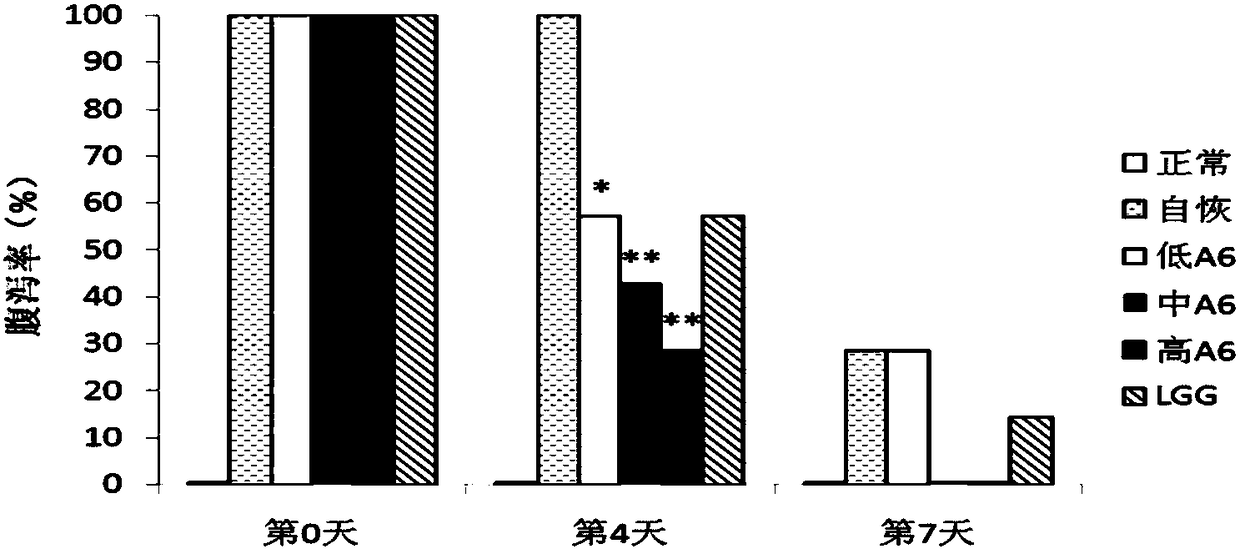
[0146] Example 2 Effect of Bifidobacterium A6 on AAD Mice Diarrhea Index
[0147] 2.1 Changes in the diarrhea rate of mice in different treatment groups during the recovery period
[0148] From figure 2 It can be seen that the diarrhea of the mice in the natural recovery group improved slowly, and 30% of the mice still had mild diarrhea on the seventh day. After intragastric administration of Bifidobacterium A6, the recovery of the mice with diarrhea was accelerated, and the high-dose A6 group had the best effect. Only 30% of the mice still had symptoms of mild diarrhea on the fourth day, and all the diarrhea recovered on the seventh day. The rate of diarrhea in mice fed with LGG was similar to that in mice fed with low doses of A6. The number of mice with diarrhea was reduced by about half on the fourth day of the experiment, and some mice still had diarrhea on the seventh day.
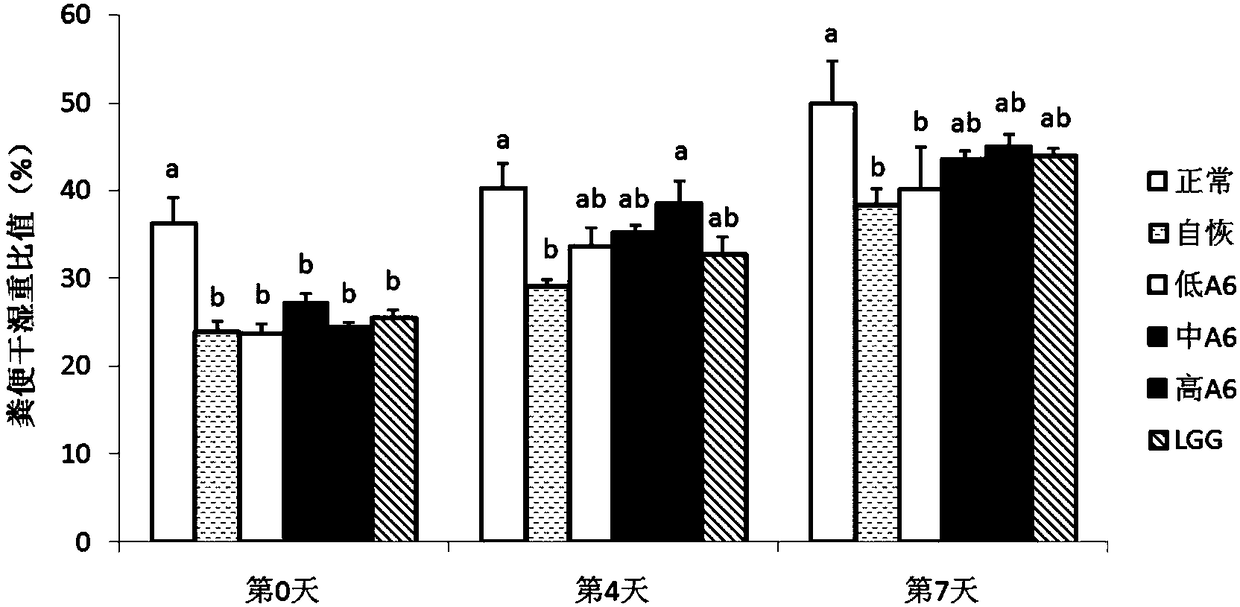
[0149] 2.2 Changes in the dry-wet weight ratio of feces in different treatment groups during...
Embodiment 3
[0151] Example 3 The effect of bifidobacterium A6 on the size of the cecum of AAD mice
[0152] During the dissection, it was found that the cecum of the mice treated with antibiotics was significantly enlarged, and the color was khaki, which was lighter than the dark brown of the normal group, so the weight of the cecum of the mice was detected. Such as Figure 4 shown (where, Figure 4 A is the weight of the mouse cecum, Figure 4 B is the morphological photo of the mouse cecum), the weight of the cecum in the normal group is about 0.25g, and the weight of the cecum and its contents increases to about 1g after antibiotic treatment, although supplementing high doses of A6 can reduce the weight of the cecum, which is significantly compared with the natural recovery group decreased (P<0.05), but this difference with normal mice still existed (P<0.05).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com