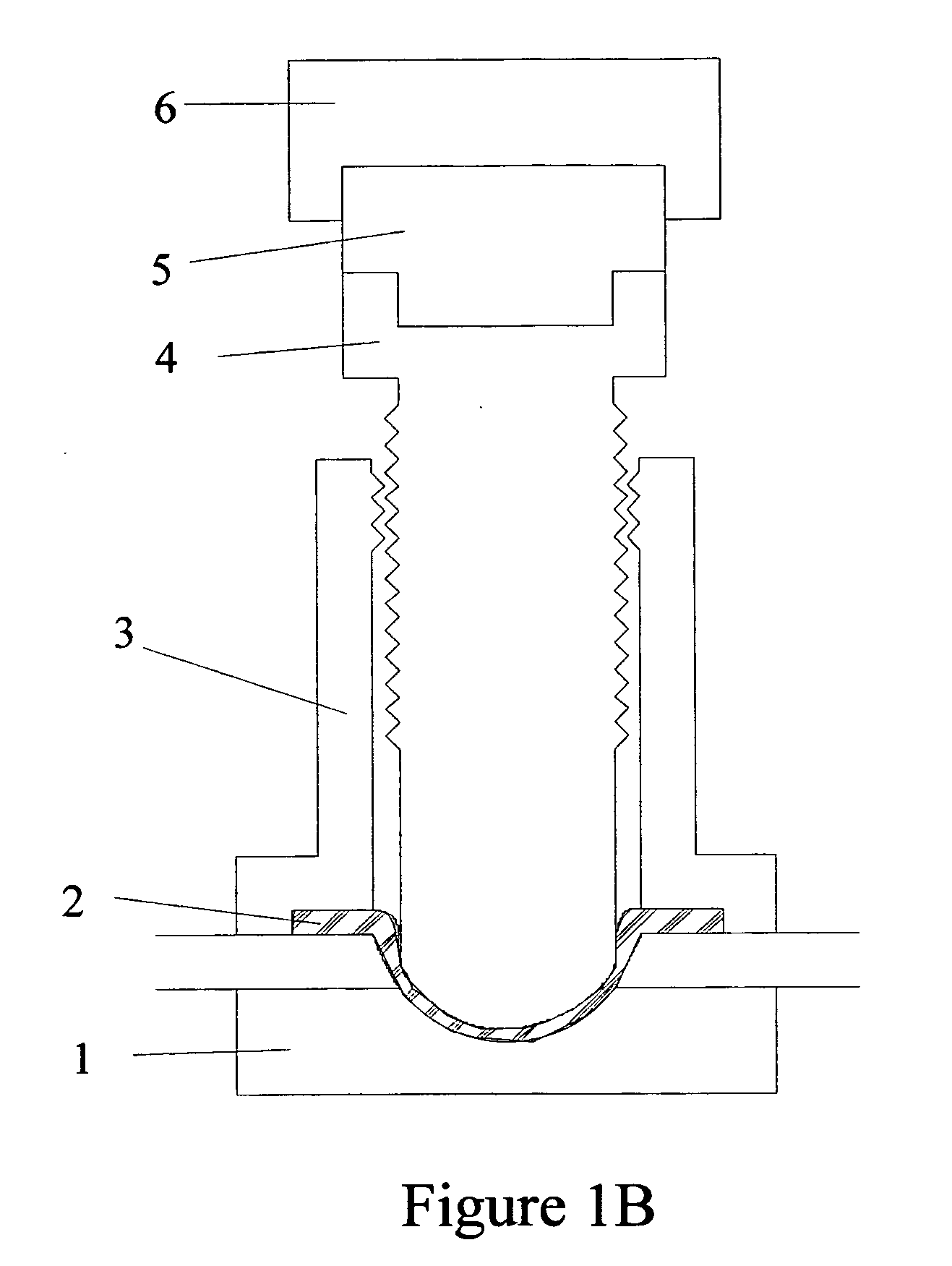
Patents
Literature
59 results about "Biotherapeutic agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein, enzyme, metabolite, nucleic acid, microorganism etc that has therapeutic characteristics; originated as a biological product but may be engineered to produce optimal therapeutic value; may include synthetic mimics.
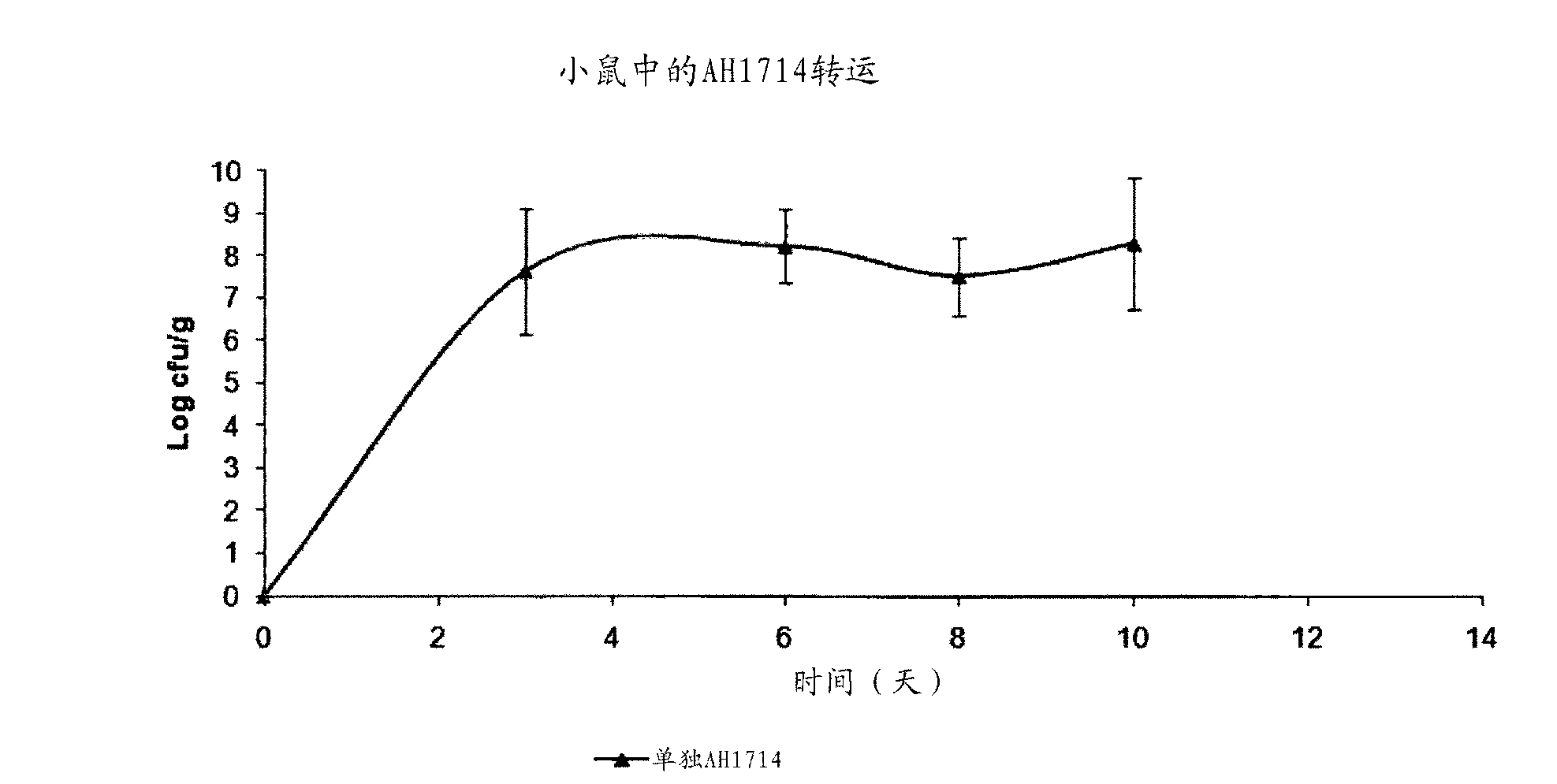
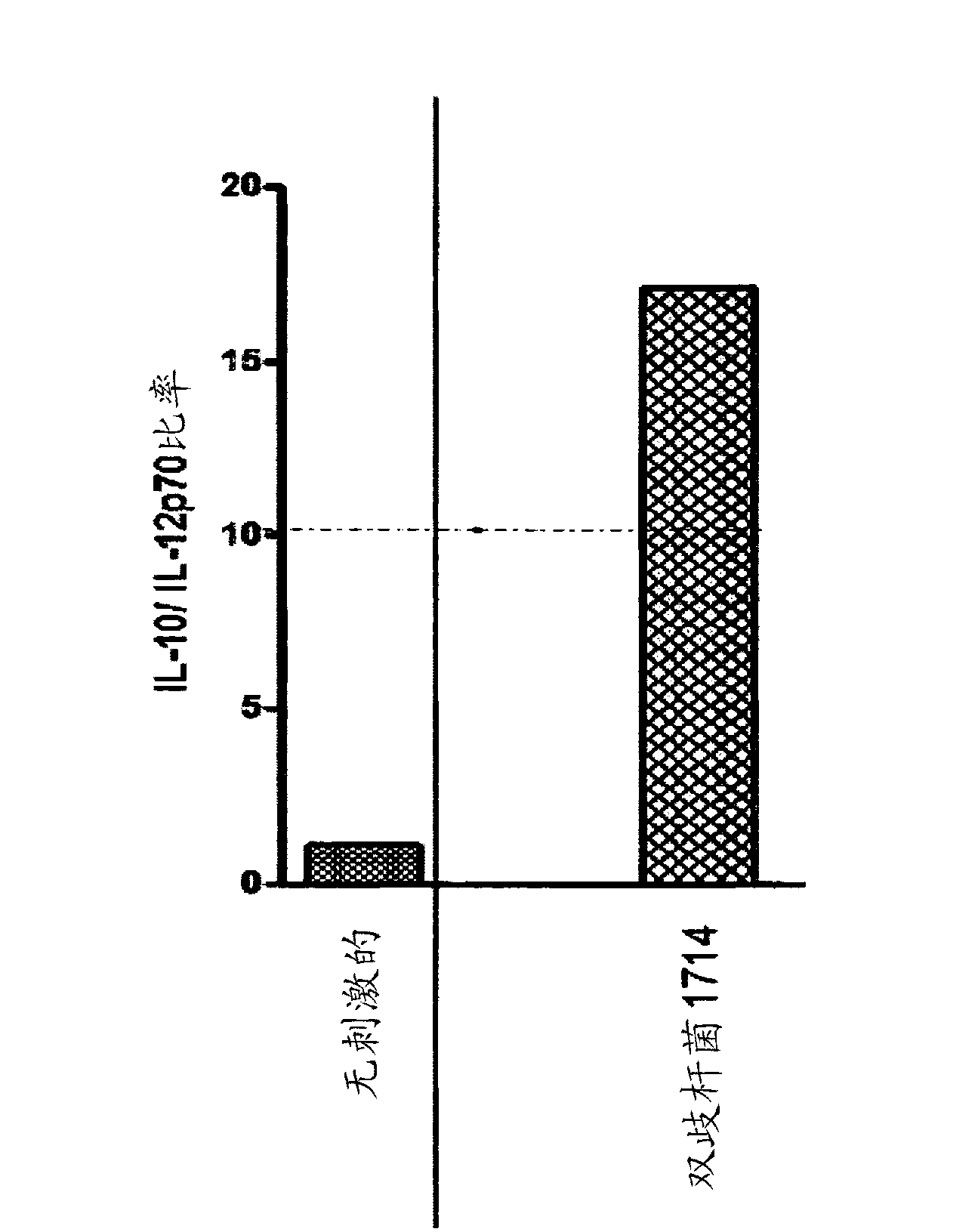
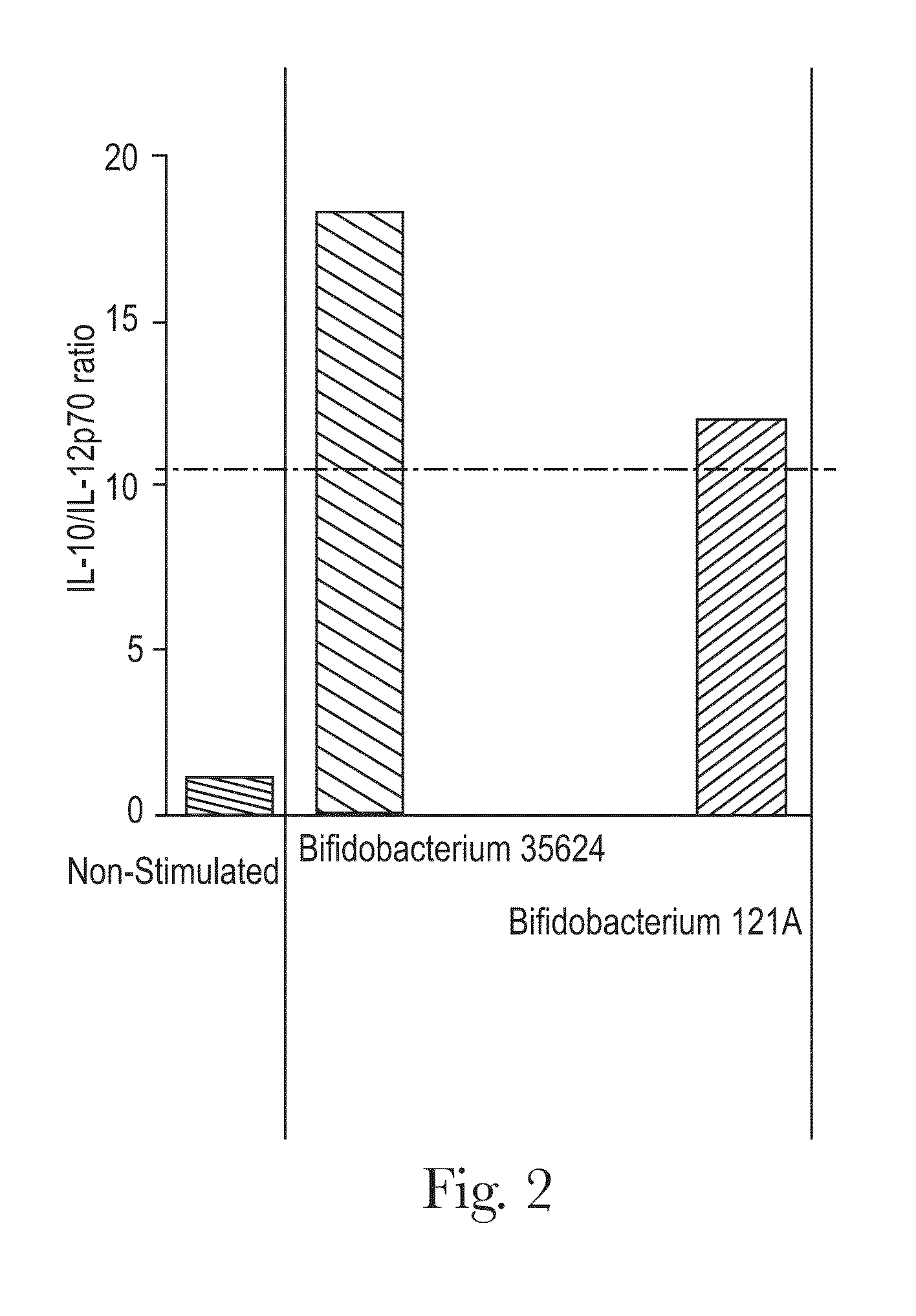
Probiotic bifidobacterium strain
Probiotic Bifidobacterium strain AH1714 is significantly immunomodulatory following oral consumption. The strain is useful as an immunomodulatory biotherapeutic agent.
Owner:PRECISIONBIOTICS GRP LTD
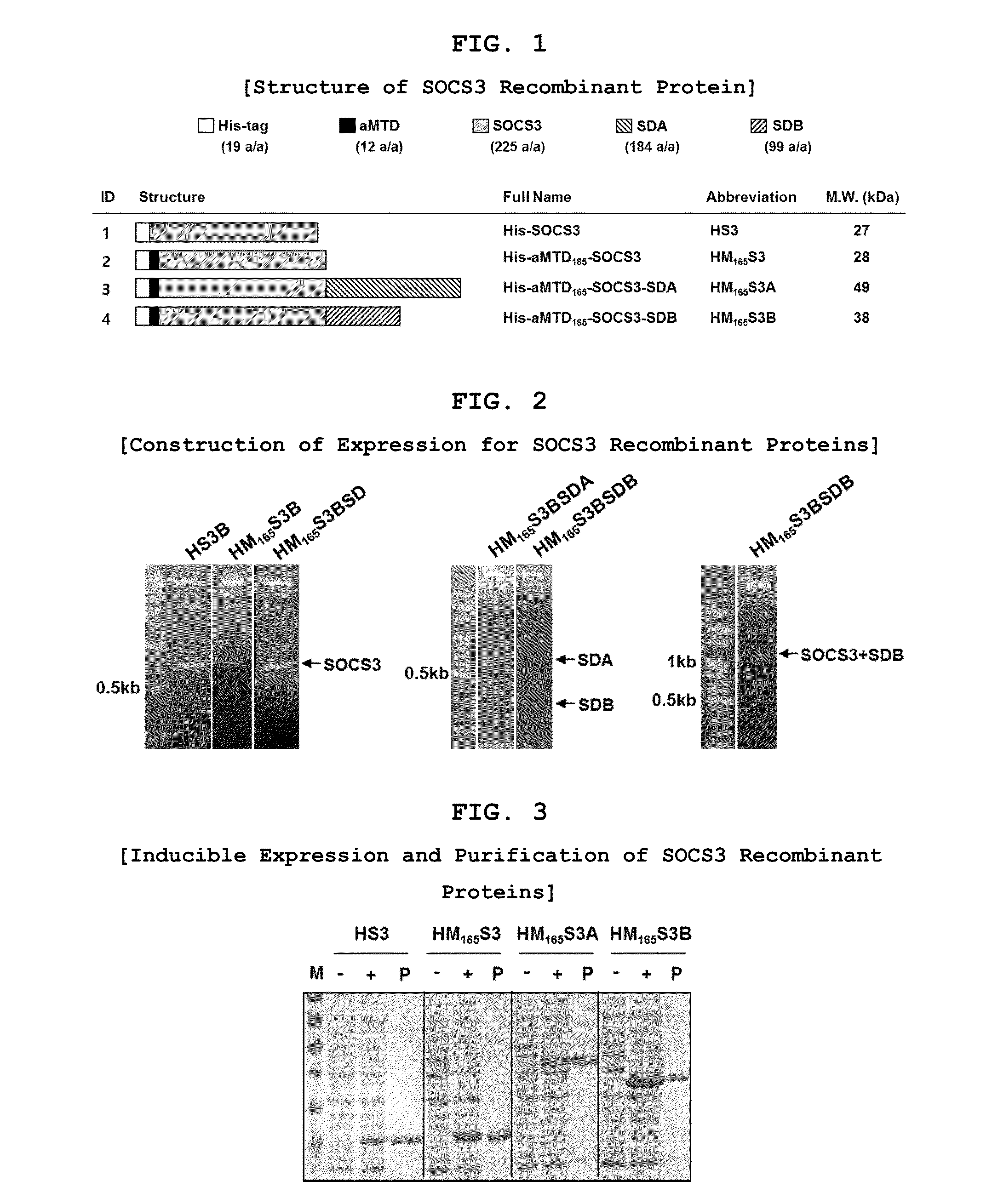
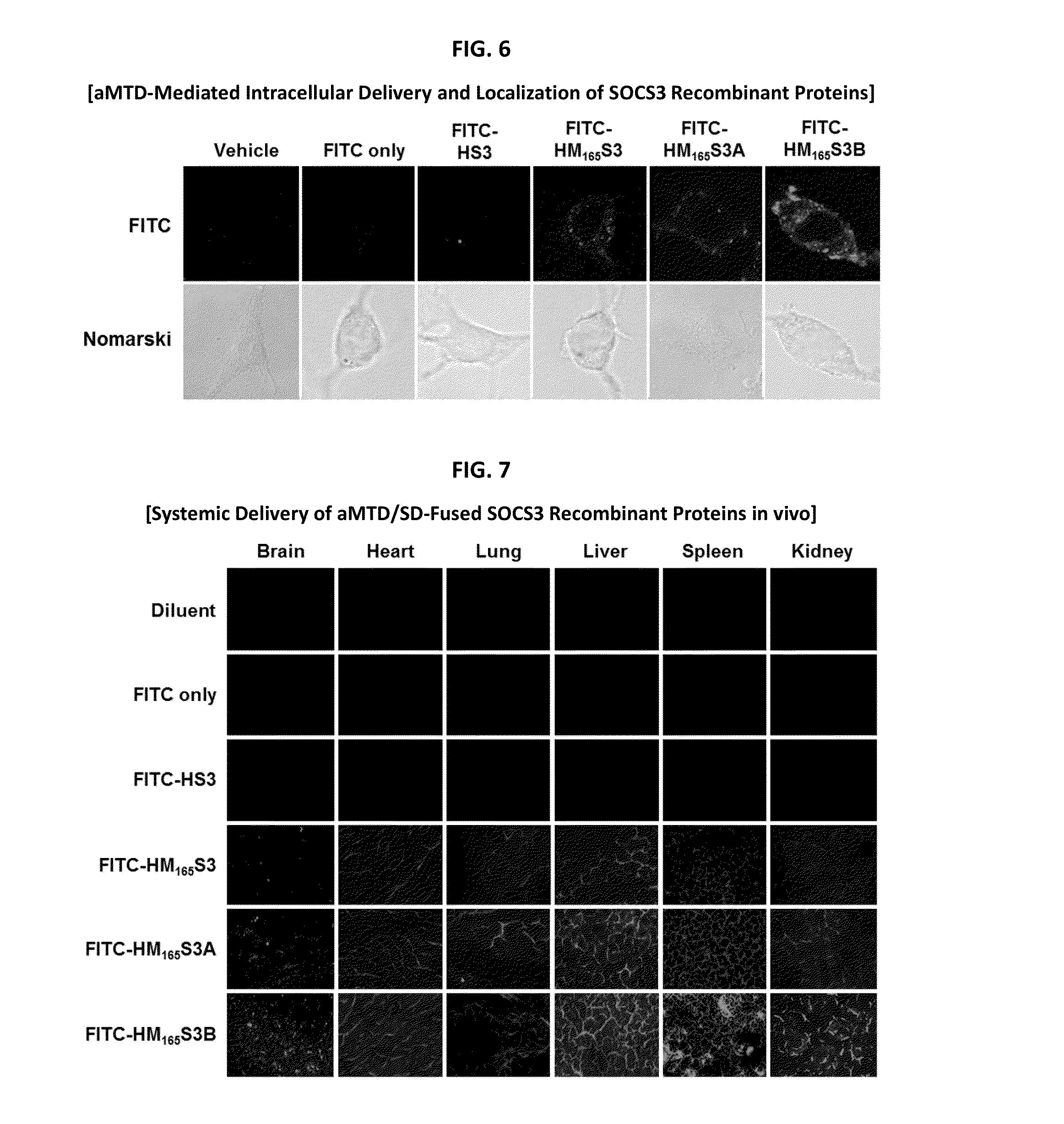
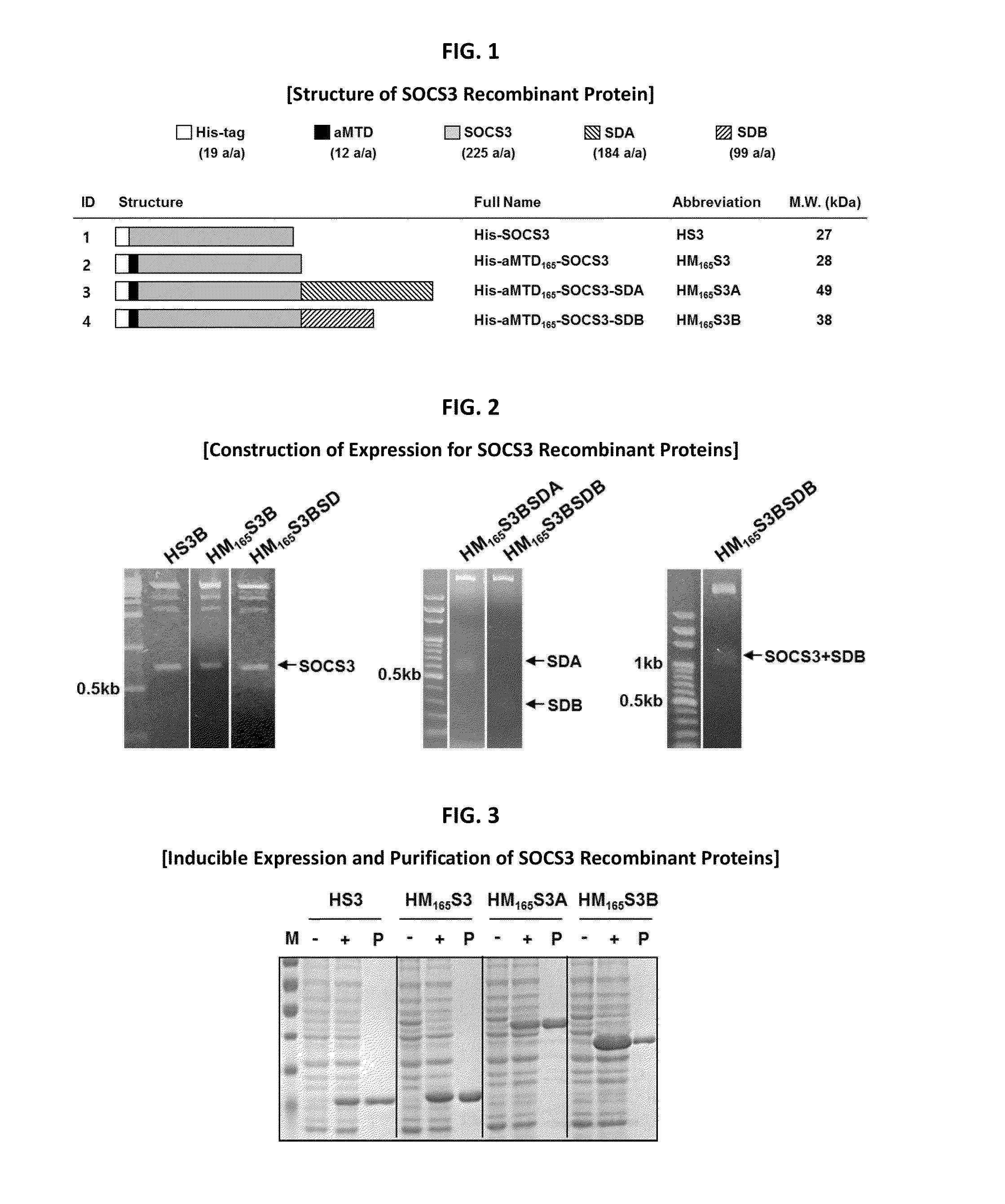
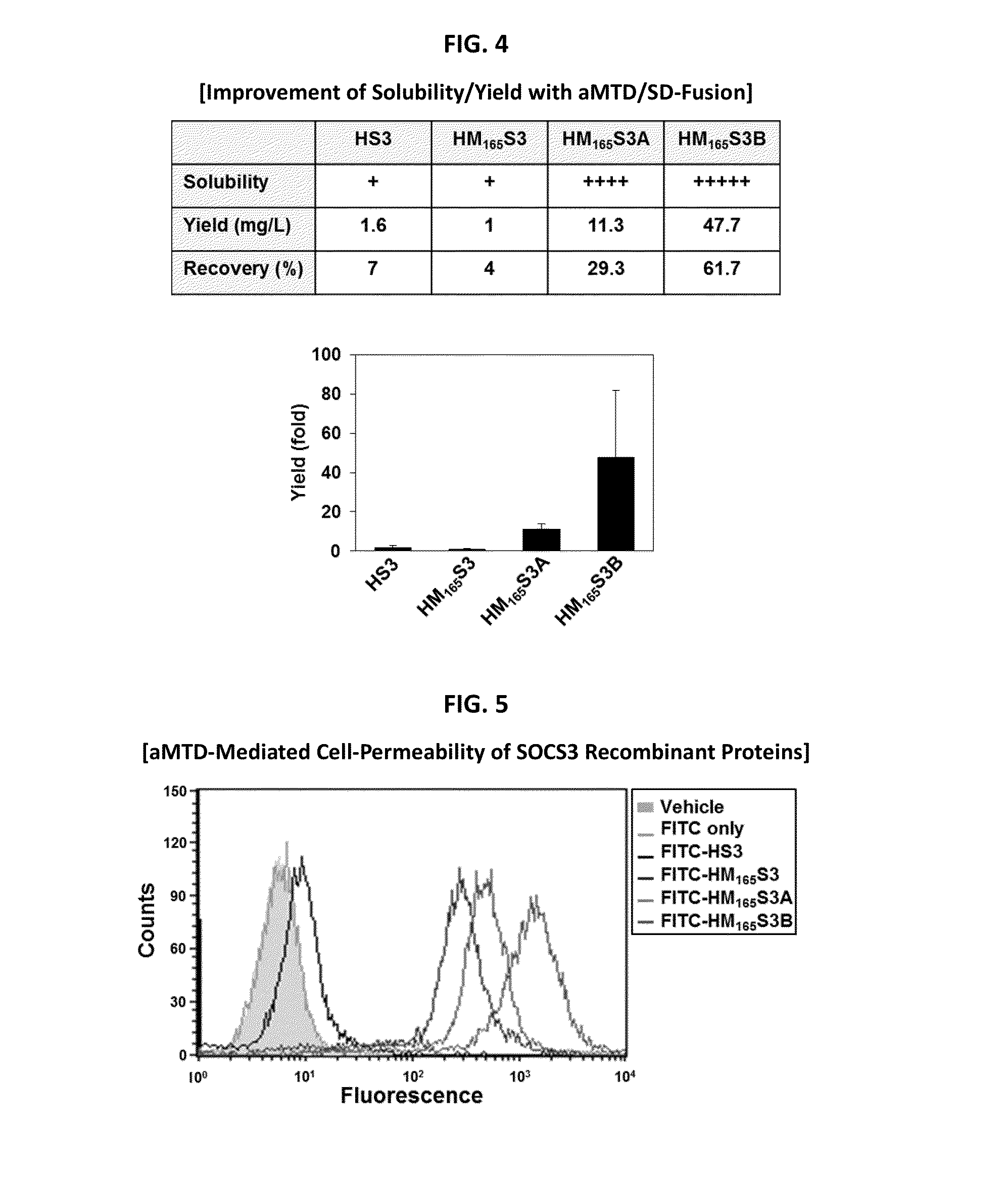
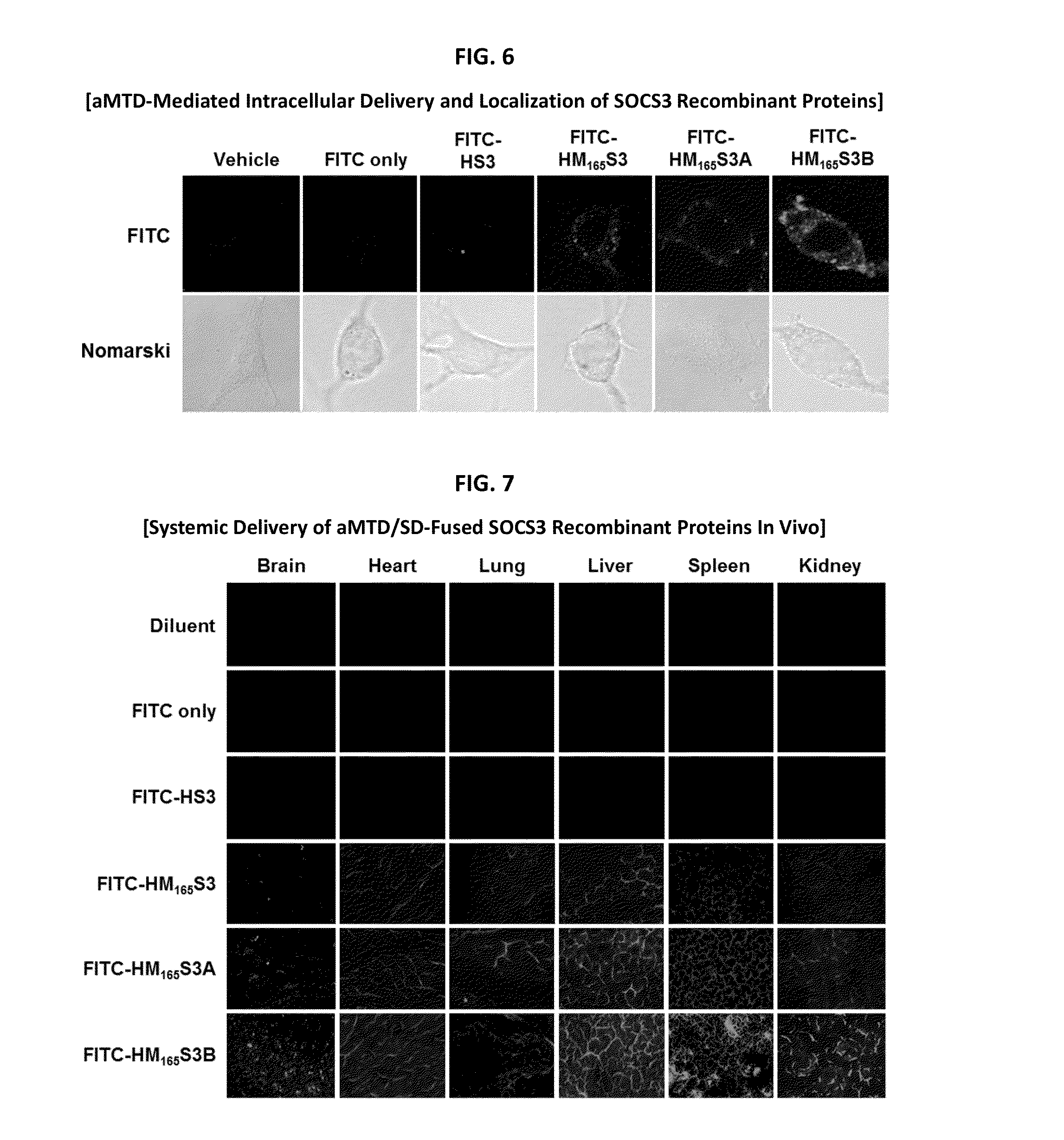
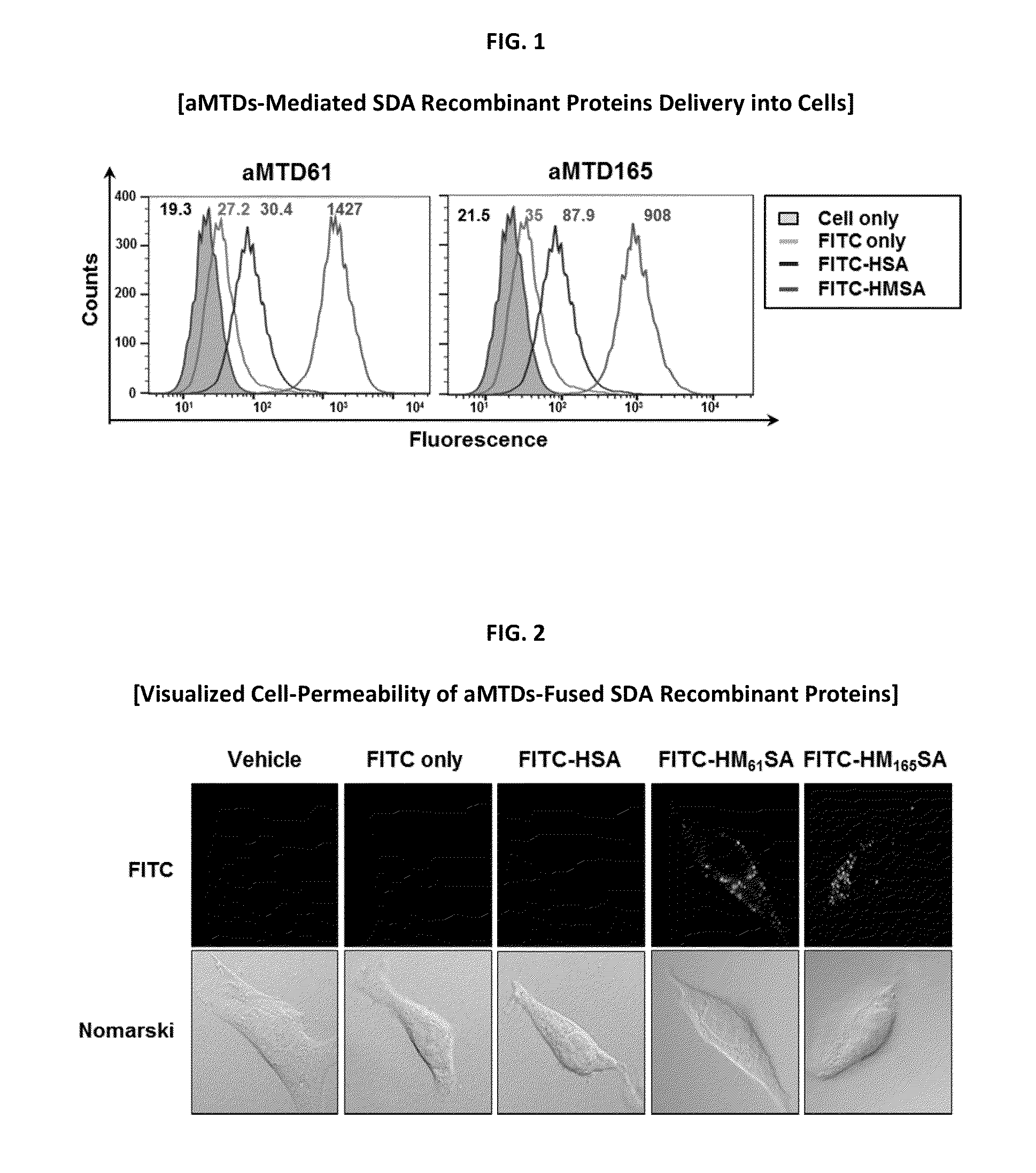
Development of Protein-Based Biotherapeutics That Penetrates Cell-Membrane and Induces Anti-Hepatocellular Carcinoma Effect - Improved Cell-Permeable Suppressor of Cytokine Signaling (iCP-SOCS3) Proteins, Polynucleotides Encoding the Same, and Anti-Hepatocellular Carcinoma Compositions Comprising the Same
InactiveUS20160060310A1Good effectImprove efficiencyPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityHepatocellular carcinoma
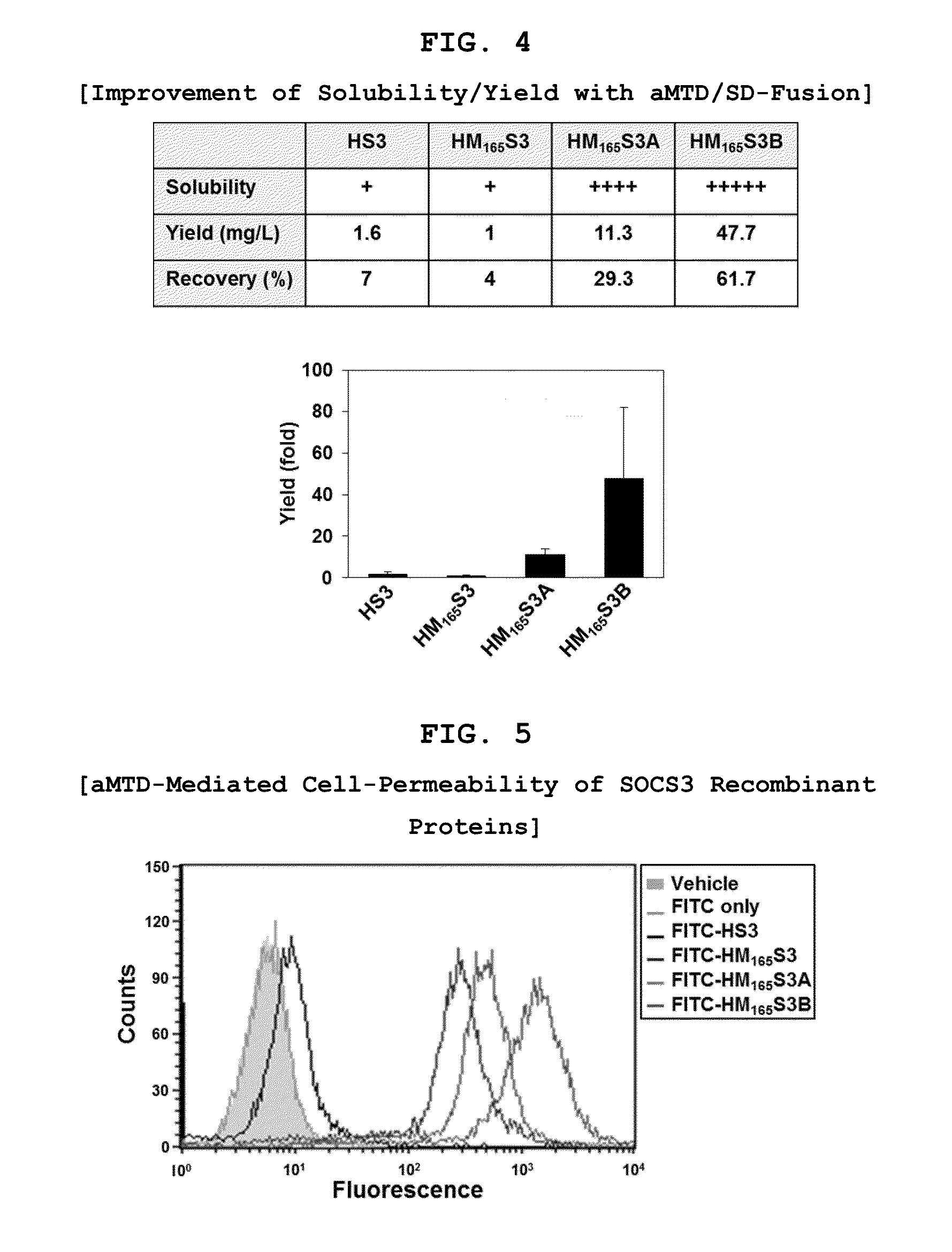
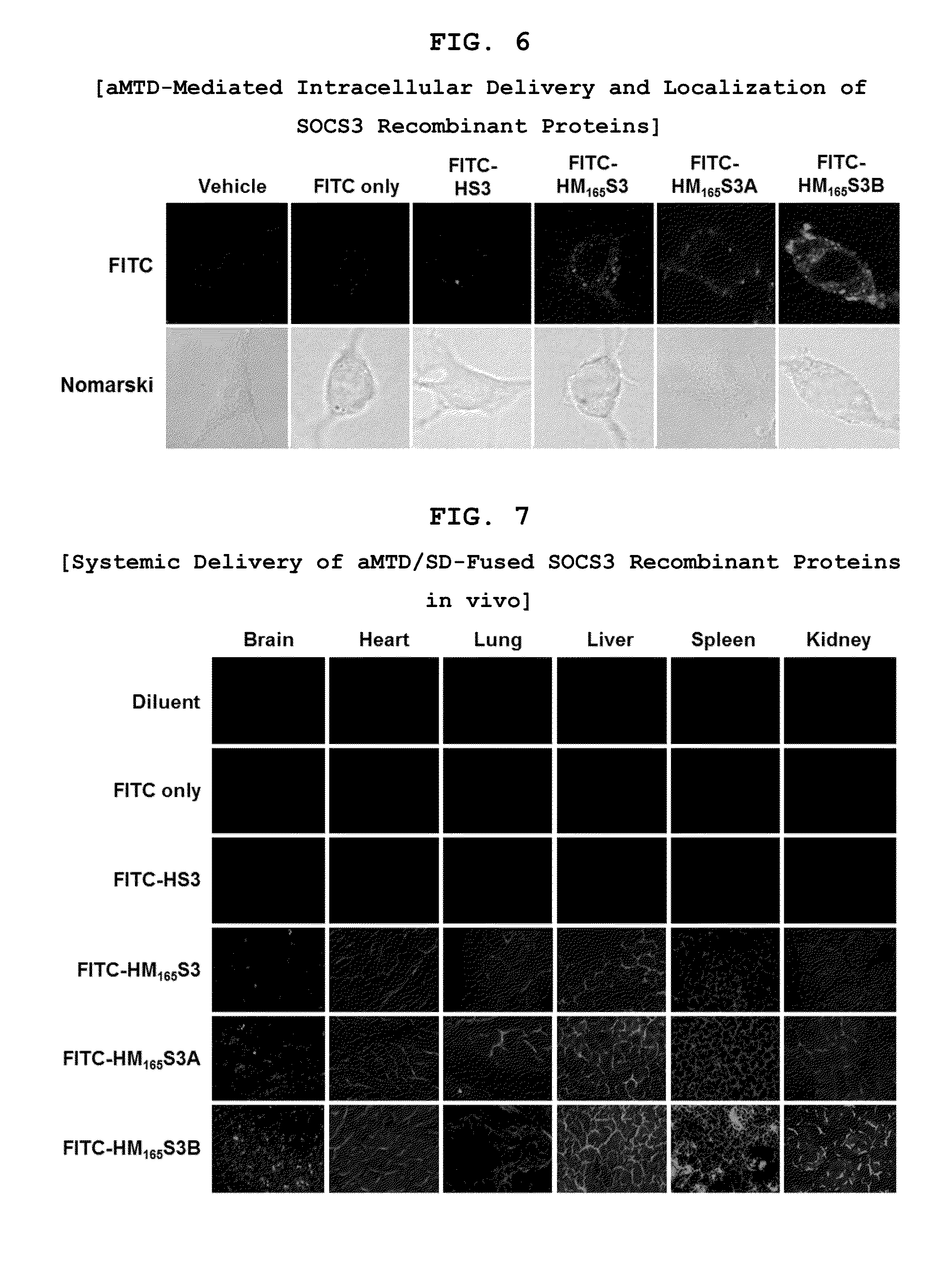
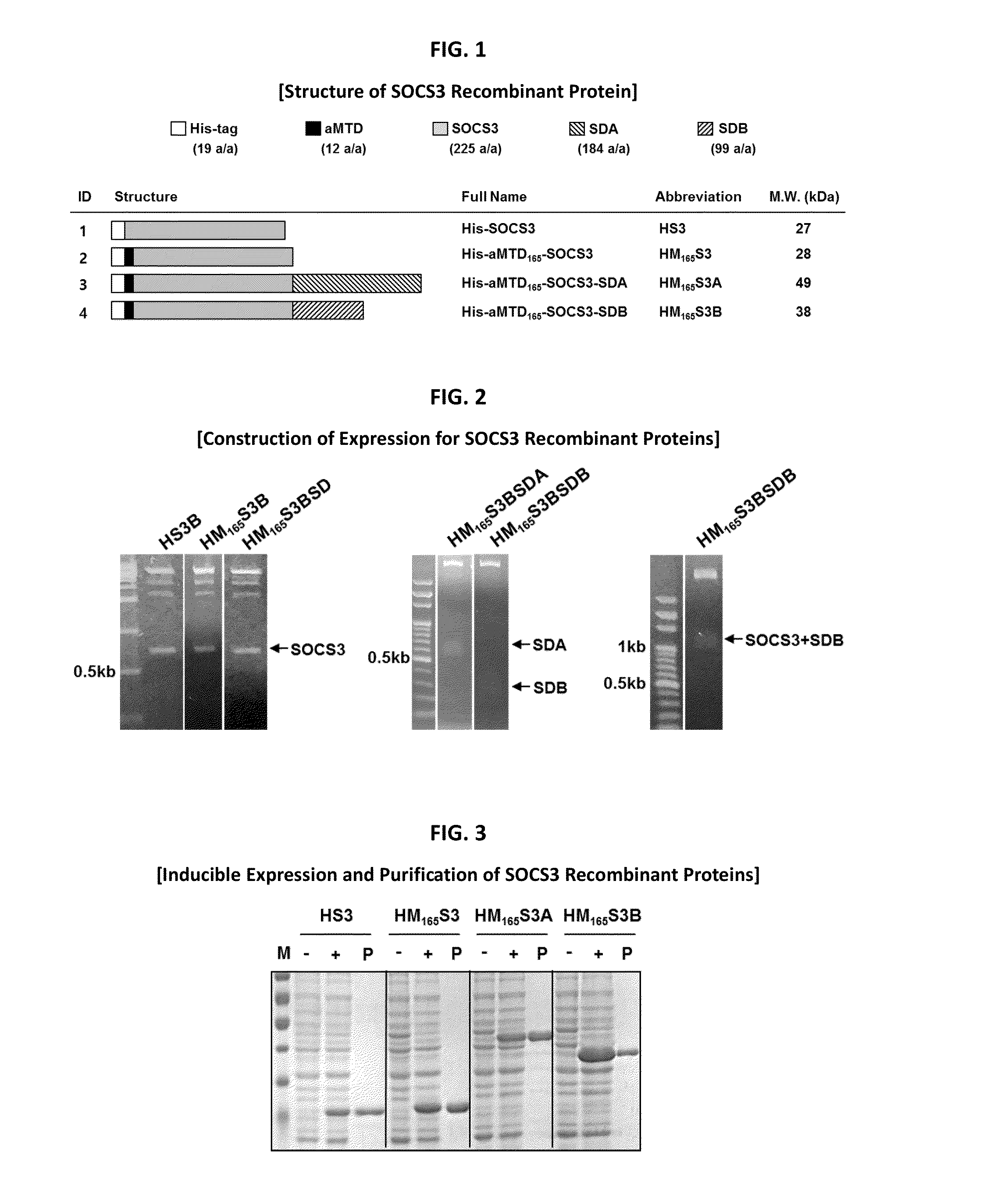
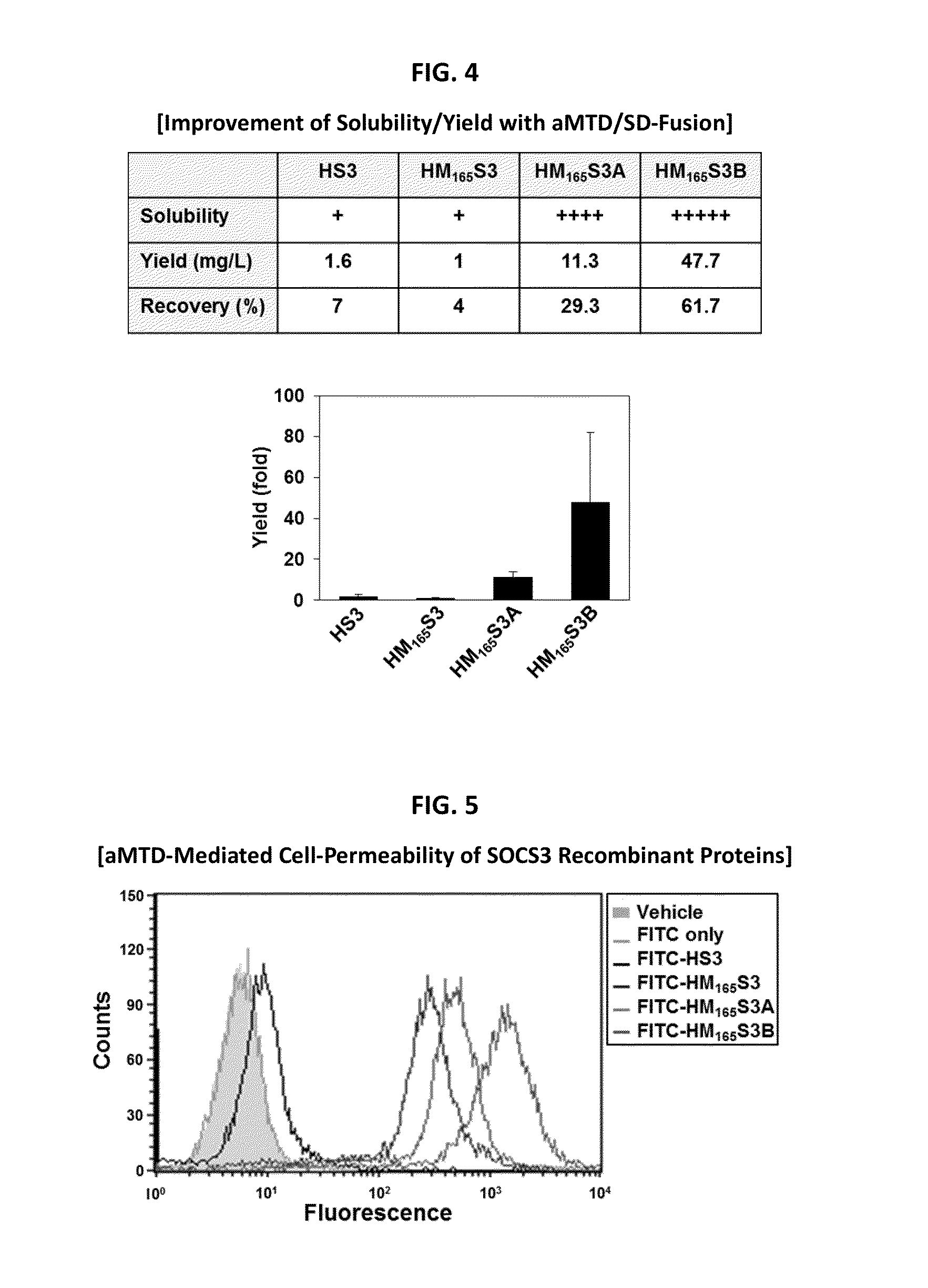
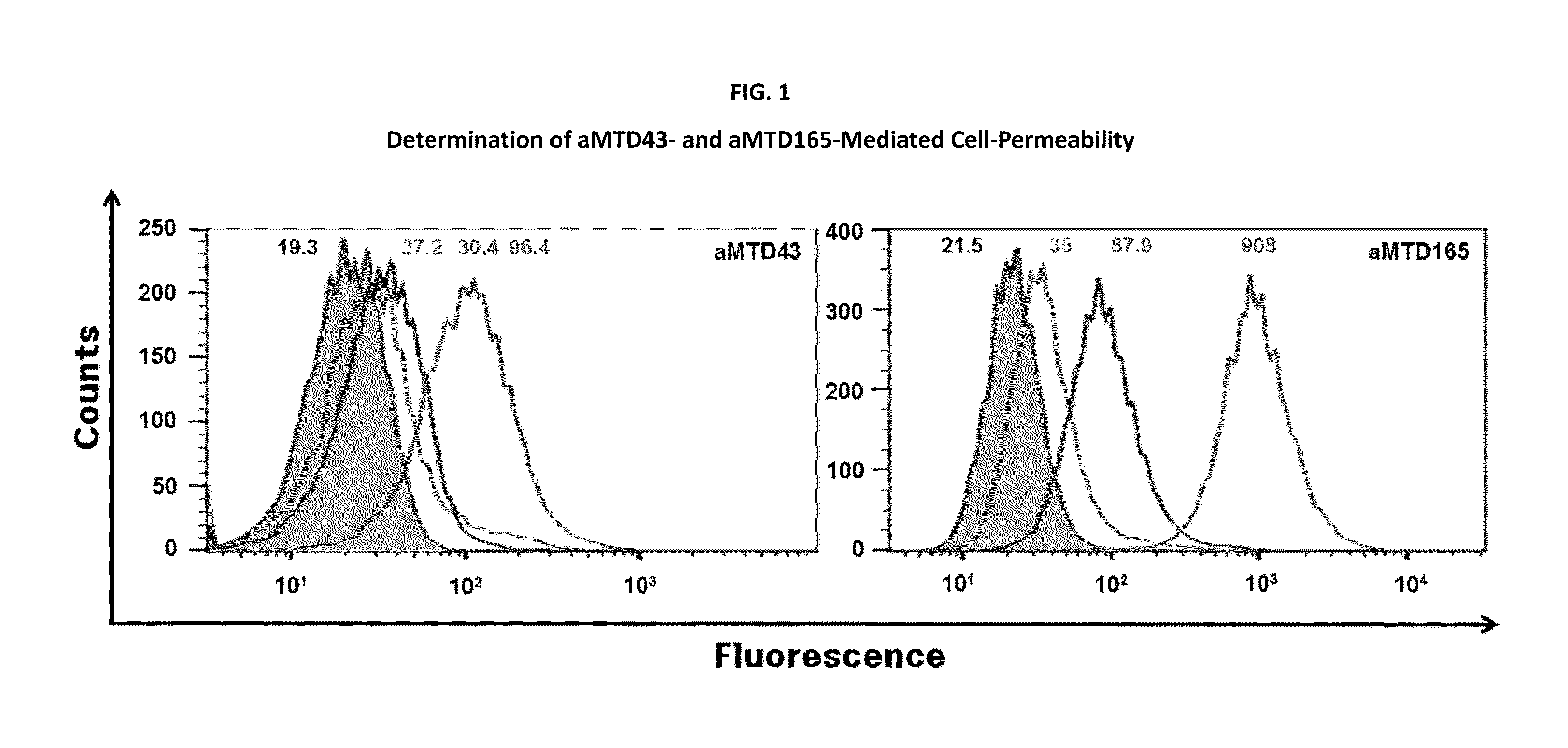
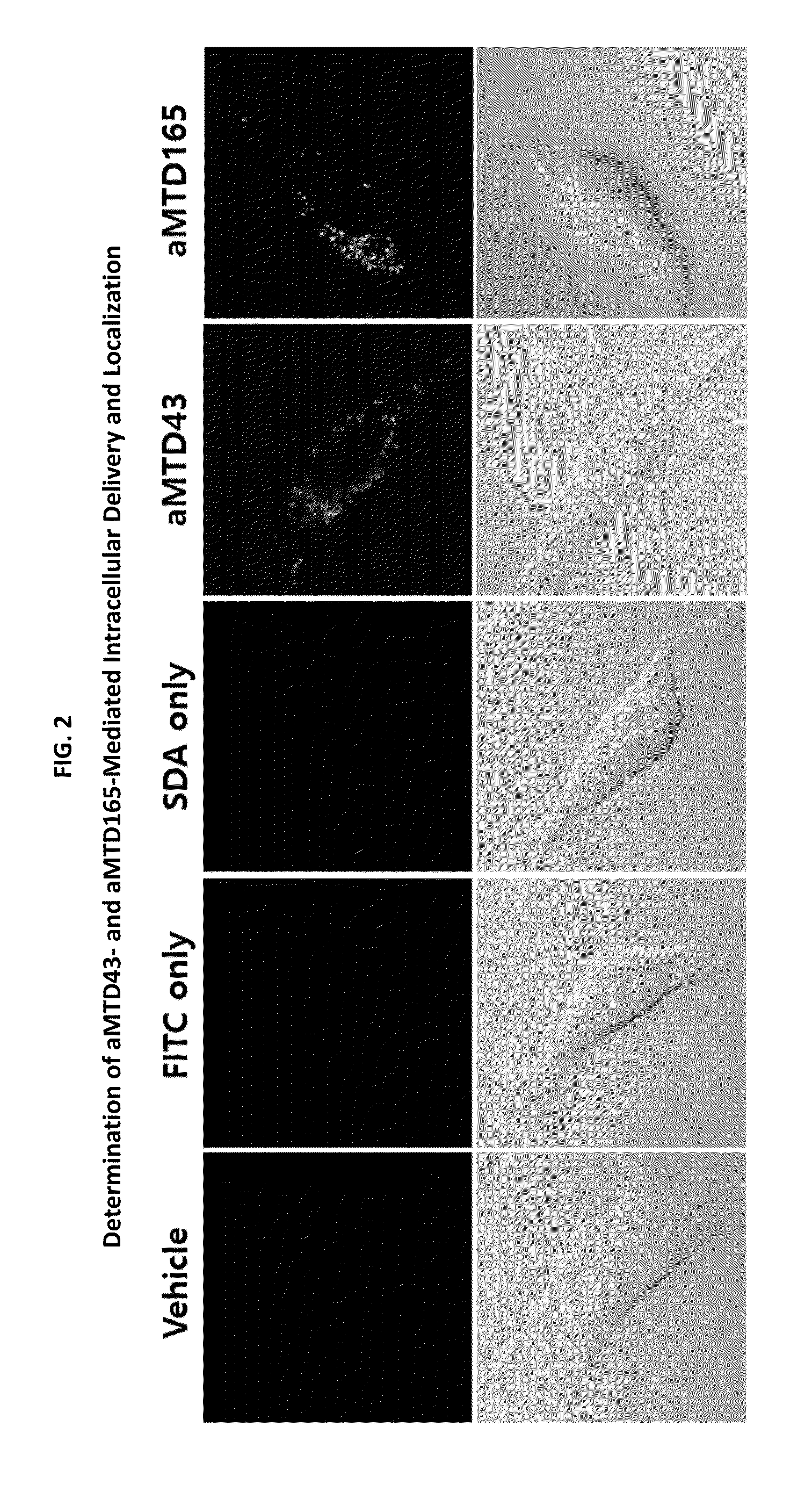
Protein transduction exploits the ability of some cell-penetrating peptide (CPP) sequences to enhance the uptake of proteins and other macromolecules by mammalian cells. Previously developed hydrophobic CPPs, named membrane translocating sequence (MTS), membrane translocating motif (MTM) and macromolecule transduction domain (MTD), are able to deliver biologically active proteins into a variety of cells and tissues. Various cargo proteins fused to these CPPs have been used to test the functional and / or therapeutic efficacy of protein transduction. For example, recombinant proteins consisting of suppressor of cytokine signaling 3 protein (CP-SOCS3) fused to the fibroblast growth factor (FGF) 4-derived MTM were developed to inhibit inflammation and apoptosis. However, CP-SOCS3 fusion proteins expressed in bacteria were hard to purify in soluble form. To address these critical limitations, CPP sequences called advanced MTDs (aMTD) have been developed in this art. This is accomplished by (i) analyzing previous developed hydrophobic CPP sequences to identify specific critical factors (CFs) that affect intracellular delivery potential and (ii) constructing artificial aMTD sequences satisfied for each critical factor. In addition, solubilization domains (SDs) have been incorporated into the aMTD-fused SOCS3 recombinant proteins to enhance solubility with corresponding increases in protein yield and cell- / tissue-permeability. These recombinant SOCS3 proteins fused to aMTD / SD having much higher solubility / yield and cell- / tissue-permeability have been named as improved cell-permeable SOCS3 (iCP-SOCS3) proteins. Previously developed CP-SOCS3 proteins fused to MTM were only tested or used as anti-inflammatory agents to treat acute liver injury. In the present art, iCP-SOCS3 proteins have been tested for use as anti-cancer agents in the treatment of hepatocellular carcinoma. Since SOCS3 is frequently deleted in and loss of SOCS3 in hepatocytes promotes resistance to apoptosis and proliferation, we reasoned that iCP-SOCS3 could be used as a protein-based intracellular replacement therapy for the treatment of hepatocellular carcinoma. The results support this reasoning: treatment of hepatocellular carcinoma cells with iCP-SOCS3 results in reduced cancer cell viability, enhanced apoptosis and loss of cell migration / invasion potential. Furthermore, iCP-SOCS3 inhibits the growth of hepatocellular carcinoma in a subcutaneous xenografts model. In the present invention with iCP-SOCS3 fused to an empirically determined combination of newly developed aMTD and customized SD, macromolecule intracellular transduction technology (MITT) enabled by the advanced MTD may provide novel protein therapy against hepatocellular carcinoma.
Owner:CELLIVERY THERAPEUTICS
Development of a Protein-Based Biotherapeutic Agent That Penetrates Cell-Membrane and Induces Anti-Tumor Effect in Solid Tumors - Improved Cell-Permeable Suppressor of Cytokine Signaling (iCP-SOCS3) Proteins, Polynucleotides Encoding the Same, and Anti-Tumor Compositions Comprising the Same
InactiveUS20160060314A1Improve anti-tumor effectImprove efficiencyPeptide/protein ingredientsAntibody mimetics/scaffoldsBiotherapeutic agentOff targets
In principle, protein-based biotherapeutics offers a way to control biochemical processes in living cells under non-steady state conditions and with fewer off-target effects than conventional small molecule therapeutics. However, systemic protein delivery in vivo has been proven difficult due to poor tissue penetration and rapid clearance. Protein transduction exploits the ability of some cell-penetrating peptide (CPP) sequences to enhance the uptake of proteins and other macromolecules by mammalian cells. Previously developed hydrophobic CPPs, named membrane translocating sequence (MTS), membrane translocating motif (MTM) and macromolecule transduction domain (MTD), are able to deliver biologically active proteins into a variety of cells and tissues. Various cargo proteins fused to these CPPs have been used to test the functional and / or therapeutic efficacy of protein transduction. The recombinant proteins consisting of suppressor of cytokine signaling 3 (CP-SOCS3) protein fused to the fibroblast growth factor (FGF) 4-derived MTM were developed to inhibit inflammation and apoptosis. However, CP-SOCS3 fusion proteins expressed in bacteria cells were hard to be purified in soluble form. To address these critical limitations, CPP sequences called advanced MTDs (aMTDs) have been developed in this art. This is accomplished by (i) analyzing previous developed hydrophobic CPP sequences to identify specific critical factors (CFs) that affect intracellular delivery potential and (ii) constructing artificial aMTD sequences satisfied for each critical factor. In addition, solubilization domains (SDs) have been incorporated into the aMTD-fused SOCS3 recombinant proteins to enhance solubility with corresponding increases in protein yield and cell- / tissue-permeability. These recombinant SOCS3 proteins fused to aMTD / SD having much higher solubility / yield and cell- / tissue-permeability have been named as improved cell-permeable SOCS3 (iCP-SOCS3) proteins. Previously developed CP-SOCS3 proteins fused to MTM were only tested or used as anti-inflammatory agents to treat acute liver injury. In the present art, iCP-SOCS3 proteins have been tested for use as anti-cancer agents in the treatment of various cancers likes gastric, colorectal and breast cancer, and glioblastoma. Since SOCS3 is frequently deleted in and loss of SOCS3 in tumors promotes resistance to apoptosis and proliferation, we reasoned that iCP-SOCS3 could be used as a protein-based intracellular replacement therapy for the treatment of various cancers. The results demonstrated in this art support the reasoning: treatment of cancer cells with iCP-SOCS3 results in reduced cancer cell viability, enhanced apoptosis of solid tumors including gastric, colorectal and breast cancer, and glioblastoma and loss of cell migration / invasion potential. Furthermore, iCP-SOCS3 inhibits the growth of gastric and colorectal tumors in a subcutaneous xenografts model. In the present invention with iCP-SOCS3, where SOCS3 is fused to an empirically determined combination of newly developed aMTD and customized SD, macromolecule intracellular transduction technology (MITT) enabled by the advanced MTDs may provide novel protein therapy against various tumors such as gastric cancer, colorectal cancer, glioblastoma, and breast cancer.
Owner:JO DAEWOONG +1
Development of Protein-Based Biotherapeutics That Penetrates Cell-Membrane and Induces Anti-Angiogenic Effect - Improved Cell-Permeable Suppressor of Cytokine Signaling (iCP-SOCS3) Proteins, Polynucleotides Encoding the Same, and Anti-Angiogenic Compositions Comprising the Same
InactiveUS20160060313A1Good effectImprove efficiencyPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityApoptosis
In principle, protein-based biotherapeutics offers a way to control biochemical processes in living cells under non-steady state conditions and with fewer off-target effects than conventional small molecule therapeutics. However, systemic protein delivery in vivo has been proven difficult due to poor tissue penetration and rapid clearance. Protein transduction exploits the ability of some cell-penetrating peptide (CPP) sequences to enhance the uptake of proteins and other macromolecules by mammalian cells. Previously developed hydrophobic CPPs—named membrane translocating sequence (MTS), membrane translocating motif (MTM) and macromolecule transduction domain (MTD)—are able to deliver biologically active proteins into a variety of cells and tissues. Various cargo proteins fused to these CPPs have been used to test the functional and / or therapeutic efficacy of protein transduction. Previously, recombinant proteins consisting of suppressor of cytokine signaling 3 (SOSC3) fused to the fibroblast growth factor (FGF) 4-derived MTM were developed to inhibit inflammation and apoptosis. However, this SOCS3 fusion proteins expressed in bacteria cells were hard to be purified in soluble form. To address these critical limitations, CPP sequences called advanced MTDs (aMTDs) have been developed in this art. The development of this art has been accomplished by (i) analyzing previous developed hydrophobic CPP sequences to identify specific critical factors (CFs) that affect intracellular delivery potential and (ii) constructing artificial aMTD sequences that satisfy each critical factor. Furthermore, solubilization domains (SDs) have been incorporated into the aMTD-fused SOCS3 recombinant proteins to enhance solubility with corresponding increases in protein yield and cell- / tissue-permeability. These recombinant SOCS3 proteins fused to aMTD / SD having much higher solubility / yield and cell- / tissue-permeability have been named as improved cell-permeable SOCS3 (iCP-SOCS3) proteins. Previously developed SOCS3 recombinant proteins fused to MTM were only tested or used as anti-inflammatory agents to treat acute liver injury. In the present art, iCP-SOCS3 proteins have been tested for use as anti-angiogenic agents. Since SOCS3 is known to be an endogenous inhibitor of pathological angiogenesis, we reasoned that iCP-SOCS3 could be used as a protein-based intracellular replacement therapy for inhibiting angiogenesis in tumor cells. The results demonstrated in this art support this following reasoning: Cancer treatment with iCP-SOCS3 results in reduced endothelial cell viability, loss of cell migration potential and suppressed vascular sprouting potentials. In the present invention with iCP-SOCS3, where SOCS3 is fused to an empirically determined combination of newly developed aMTD and customized SD, macromolecule intracellular transduction technology (MITT) enabled by the advanced MTDs may provide novel protein therapy against cancer cell-mediated angiogenesis.
Owner:CELLIVERY THERAPEUTICS
Therapeutic Delivery System Comprising a High Molecular Weight Peg-Like Compound
InactiveUS20080206188A1Suppresses virulence expressionEasy to operateAntibacterial agentsNervous disorderEpitheliumPolyethylene glycol
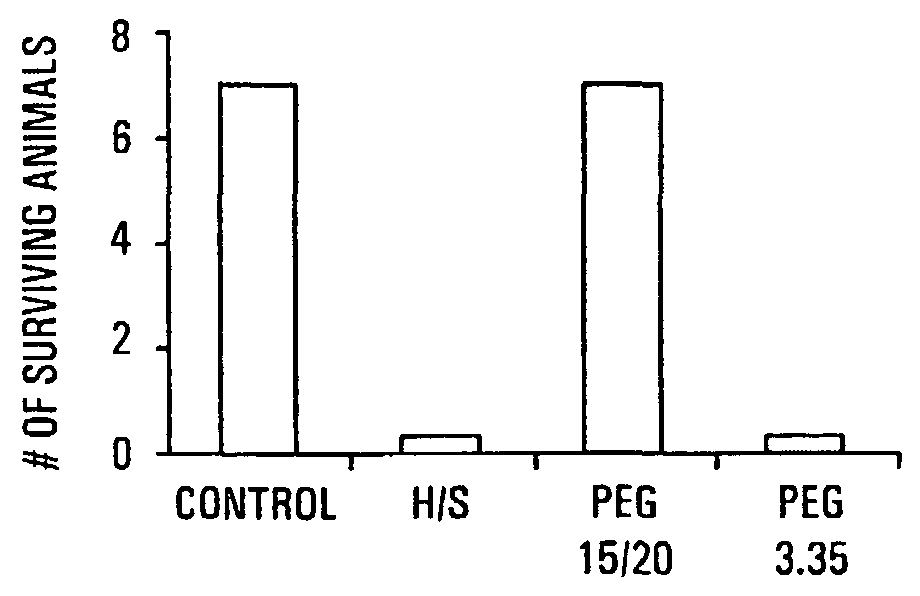
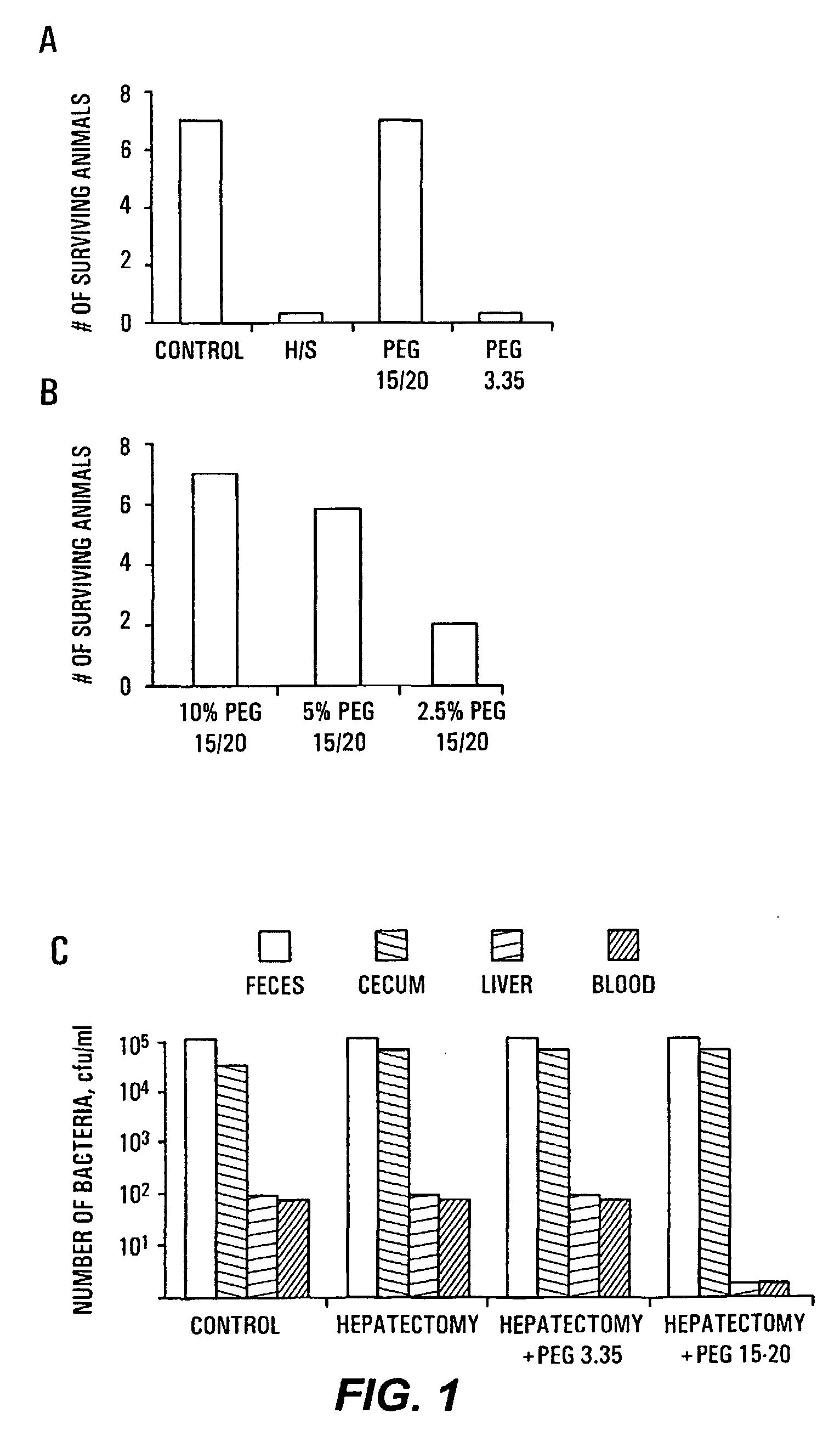
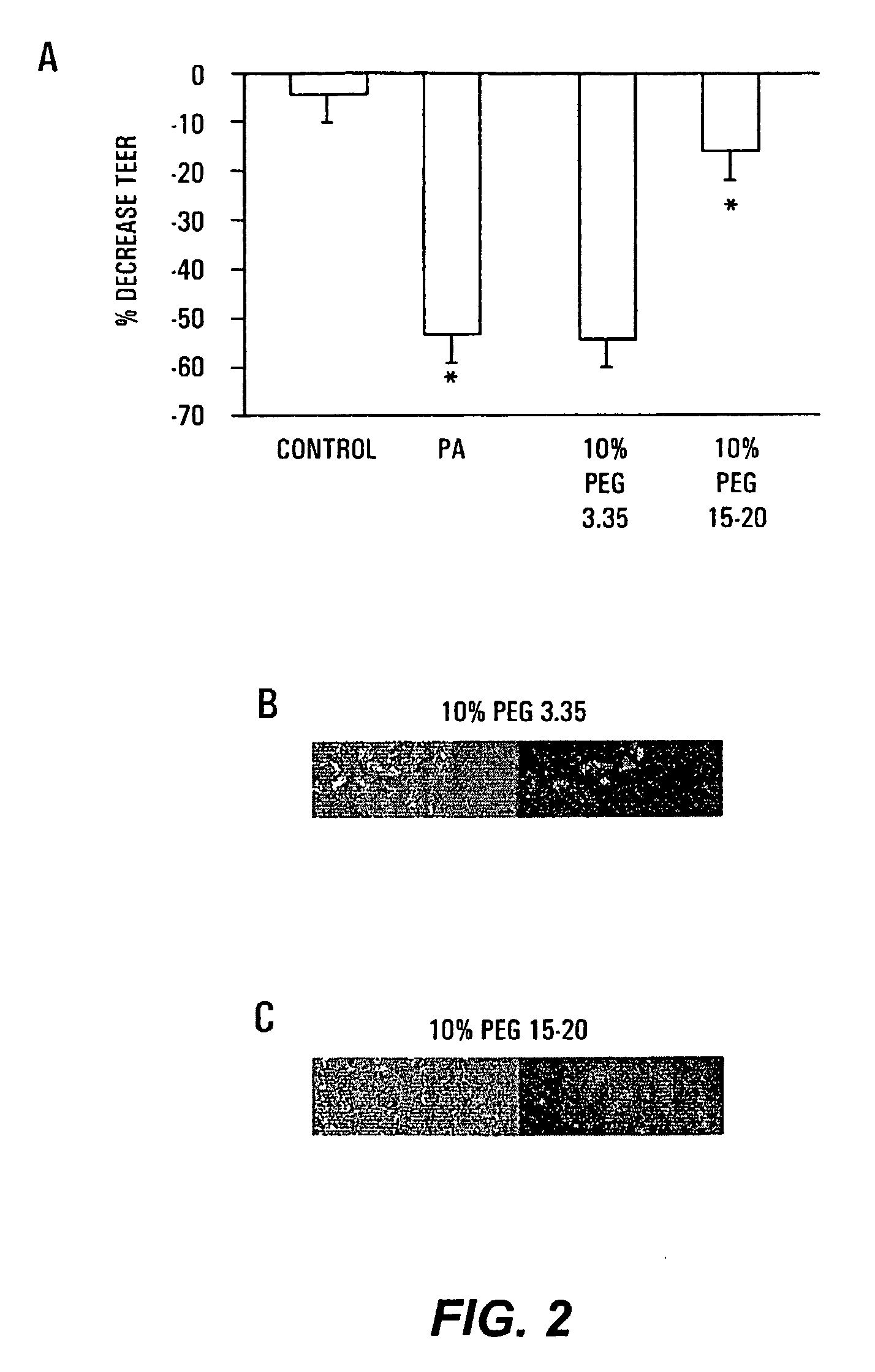
The present invention provides a system for delivering a wide range of chemical and biological therapeutics, including protein therapeutics, via transepithelial routes. The system comprises a high molecular weight polyethylene glycol-like (HMW PEG-like) compound for use with a therapeutic compound. Optionally, the system comprises a composition containing one or more HMW PEGlike compounds and one or more therapeutics, supplemented with a protective polymer such as dextran and / or essential pathogen nutrients such as L-glutamine. Administered alone, the HMW PEG-like compounds also provide therapeutic benefits. Also provided are methods for preventing or treating epithelial diseases, disorders, or conditions, such as an epithelium at risk of developing gut-derived sepsis attributable to an intestinal pathogen, as well as methods for monitoring the administration of HMW PEG-like compounds.
Owner:UNIVERSITY OF CHICAGO
Cross-screening system and methods for detecting a molecule having binding affinity for a target molecule
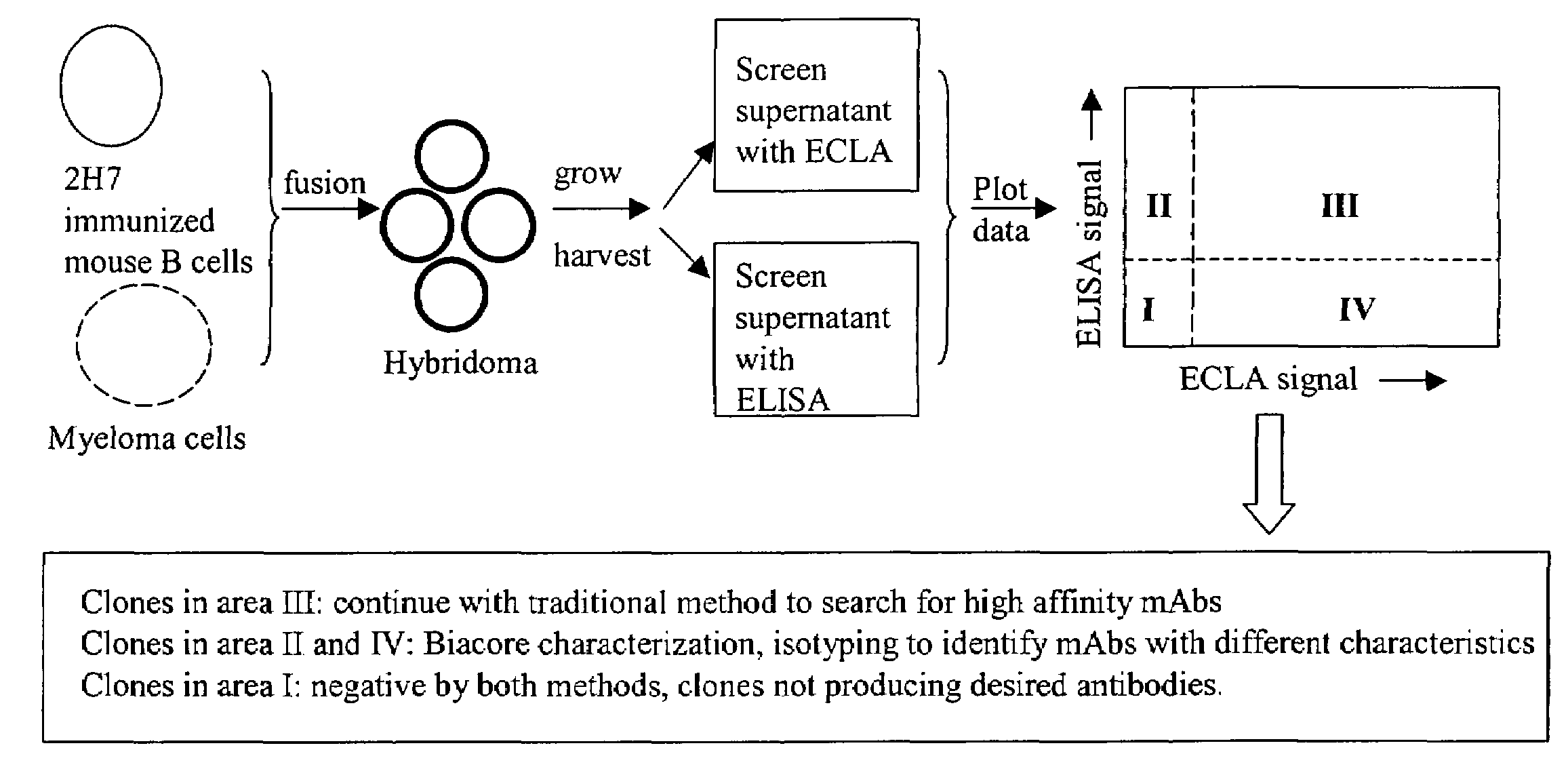
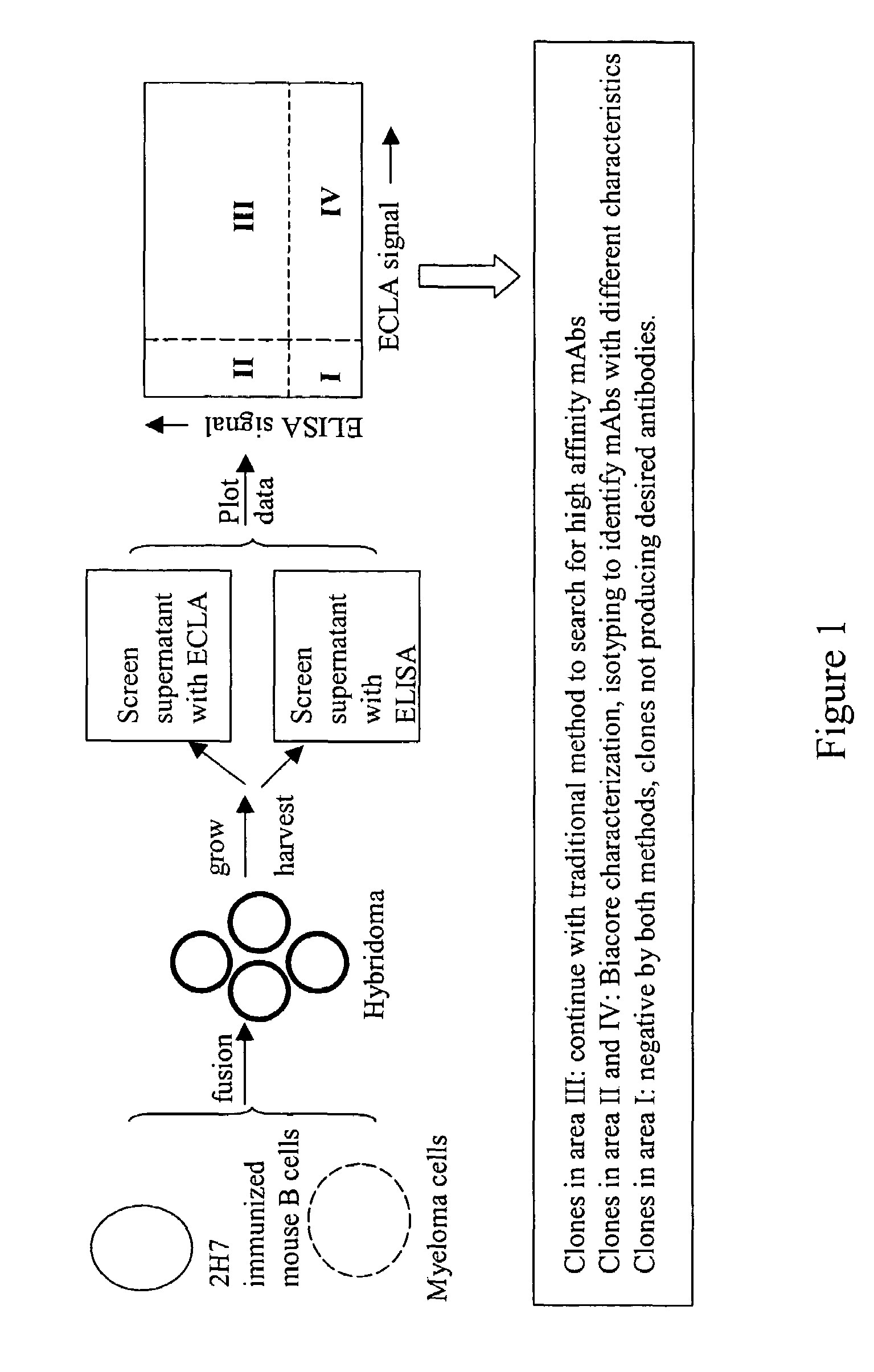
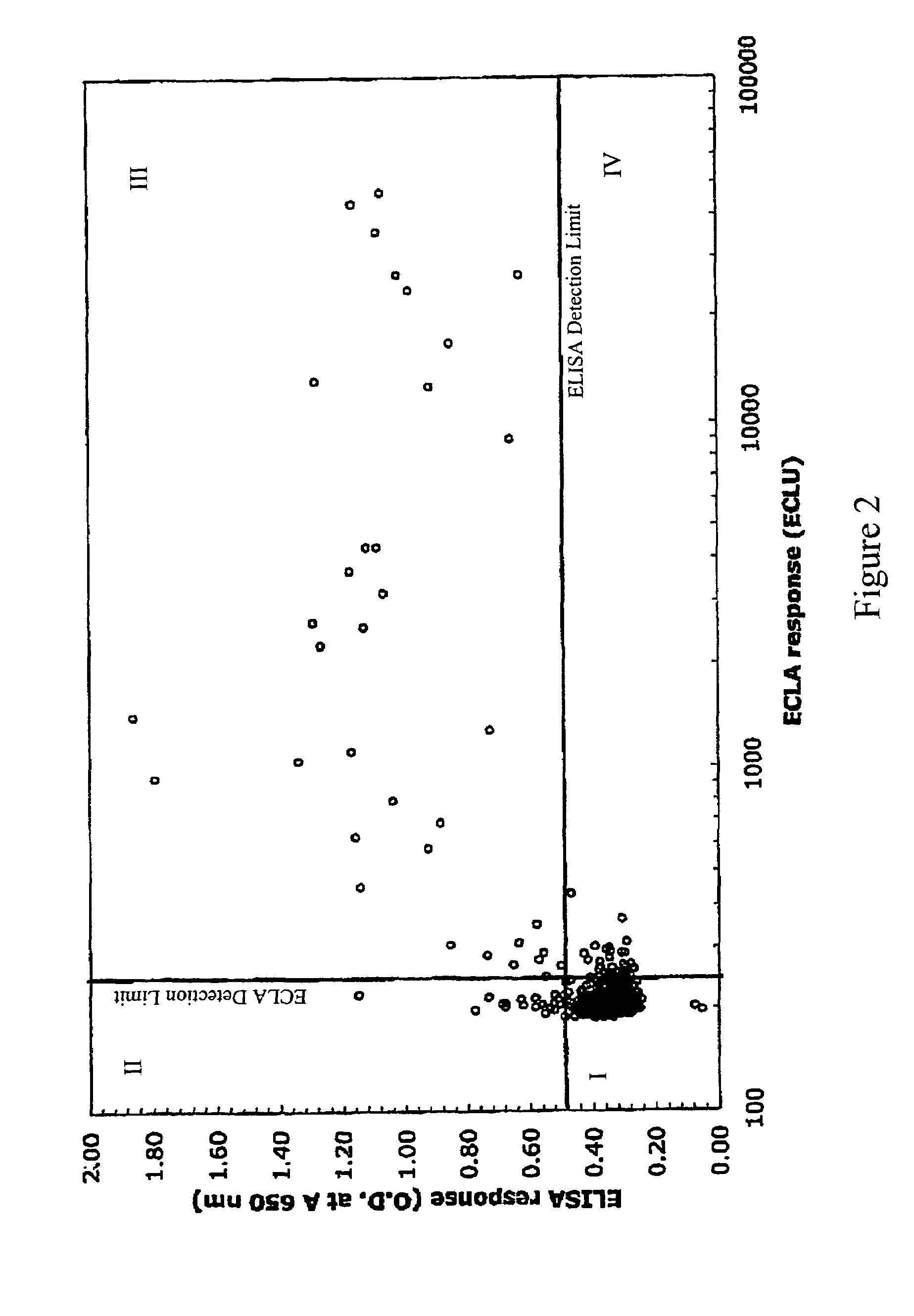
The invention is directed to a cross-screening system and methods of the invention utilizing a combination of an immunoassay (IA) and electrochemiluminescence assay (ECLA) to identify molecules that have binding affinities for a target molecule. The cross-screening system and methods of the invention can detect molecules that have binding affinities for the target molecule below the detection limits of the individual immunoassay or ECLA. The cross-screening system and methods of the invention are useful for generating a pool of candidate analyte molecules enriched in a desired characteristic, such as low binding affinity for a target molecule. Low affinity antibodies identified by the cross-screening system and methods of the invention are useful, for example, in assessing the safety and efficacy of biological therapeutics.
Owner:GENENTECH INC
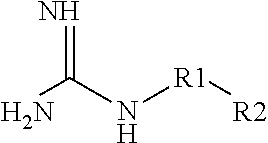
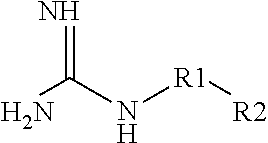
Aqueous Pharmaceutical Composition Containing A Biologic Therapeutic Agent And Guanidine Or A Guanidine Derivative And An Injection Including The Composition
ActiveUS20130317457A1Reduce aggregationLow composition viscosityOrganic active ingredientsBiocideGuanidine derivativesArginine
The present invention is directed to an aqueous pharmaceutical composition, particularly an aqueous ophthalmic composition, suitable as an injection, particularly an intravitreal injection. The composition includes a biologic therapeutic agent (e.g., an isolated monoclonal antibody that specifically binds to a C5 protein) that tends to raise the viscosity of the composition and a guanidine and / or guanidine derivative (e.g., L-arginine) that tends to lower the viscosity of the composition.
Owner:NOVARTIS AG
Diglycidic ether derivative therapeutics and methods for their use
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH +1
Torque sensitive sanitary diaphragm valves for use in the pharmaceutical industry and methods related thereto
InactiveUS20050104021A1Facilitate flow of solutionIncrease amount of necessaryDiaphragm valvesOperating means/releasing devices for valvesPharmaceutical industryDiaphragm valve
Owner:MEYERS SCOTT CHRISTOPHER
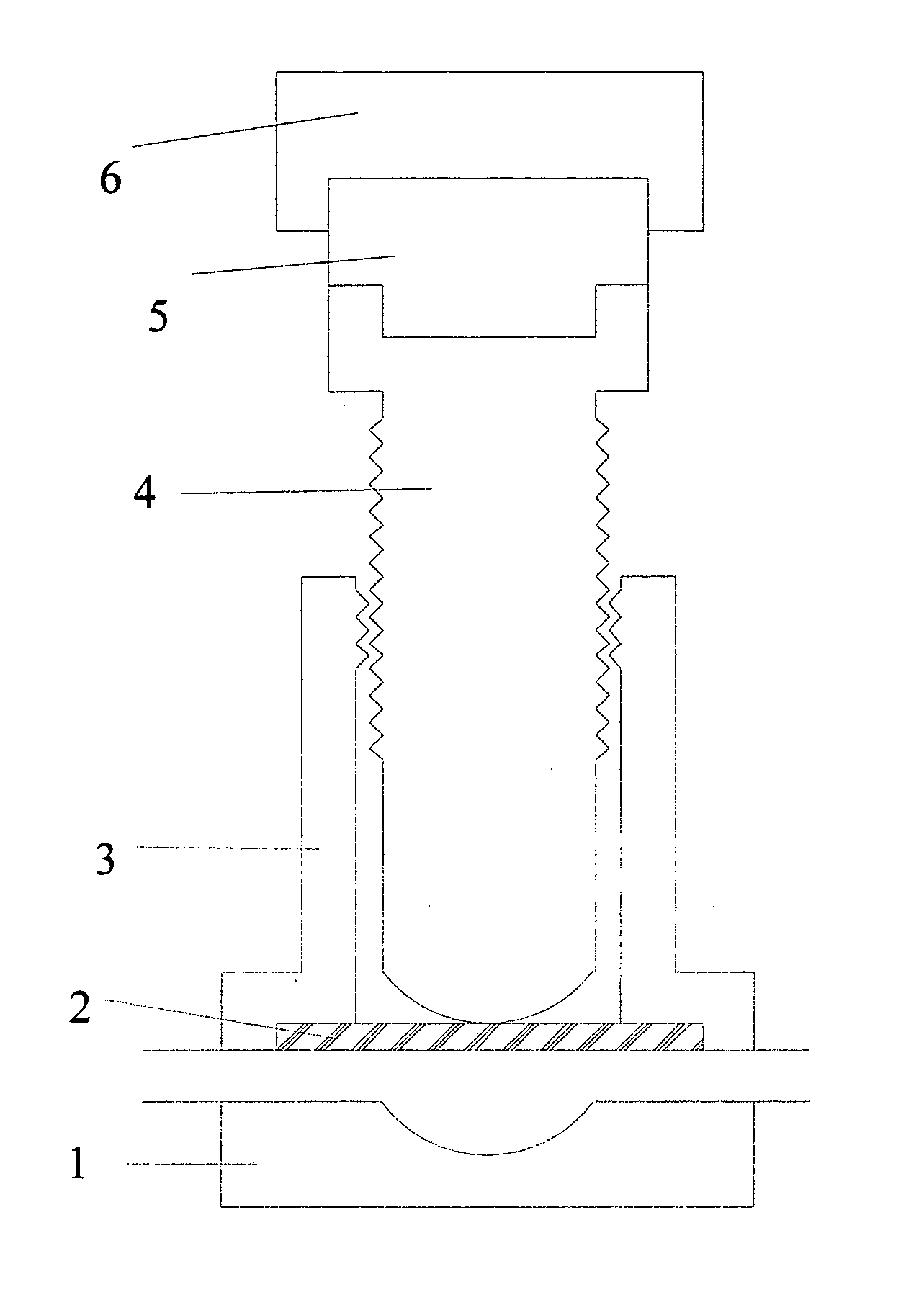
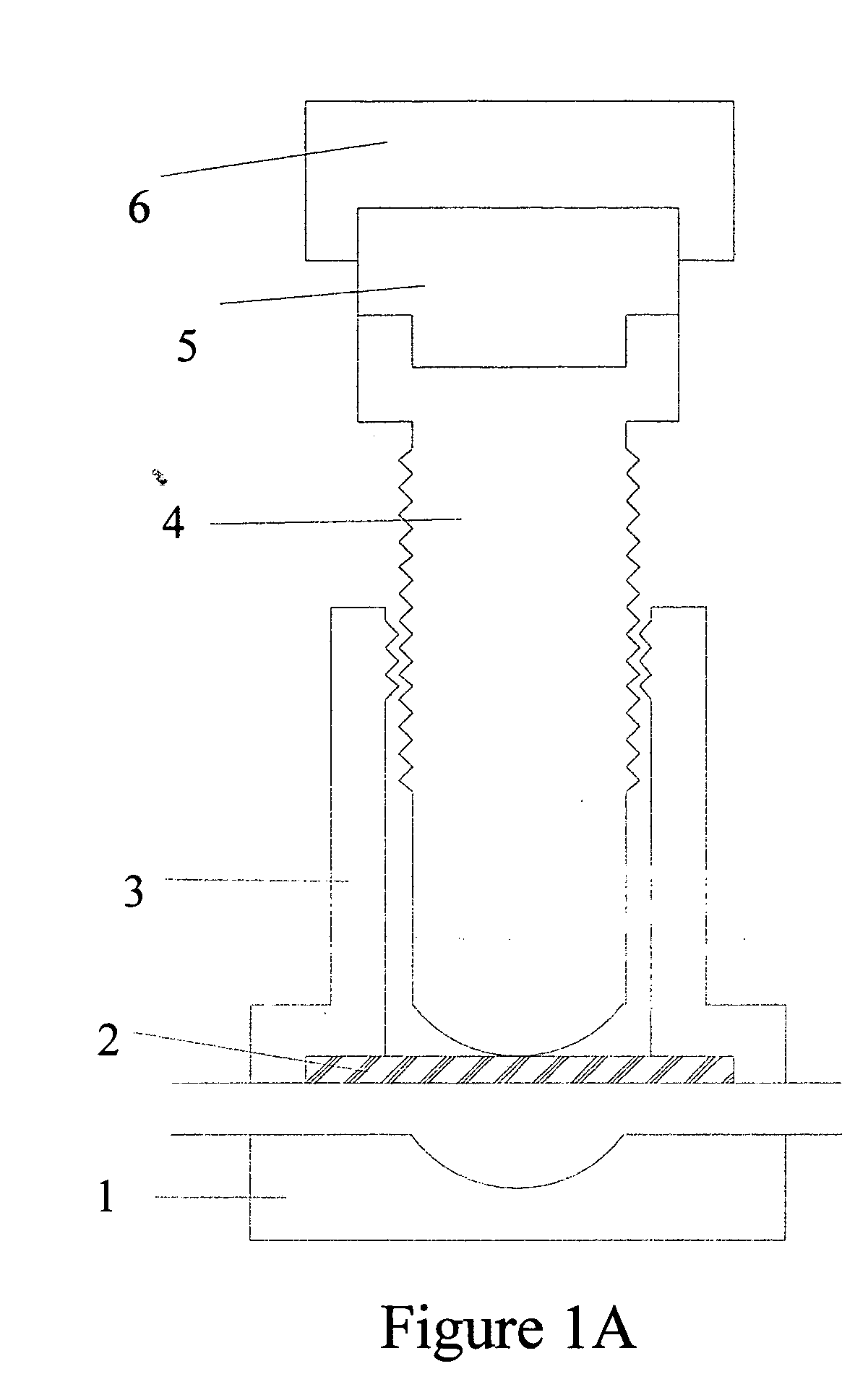
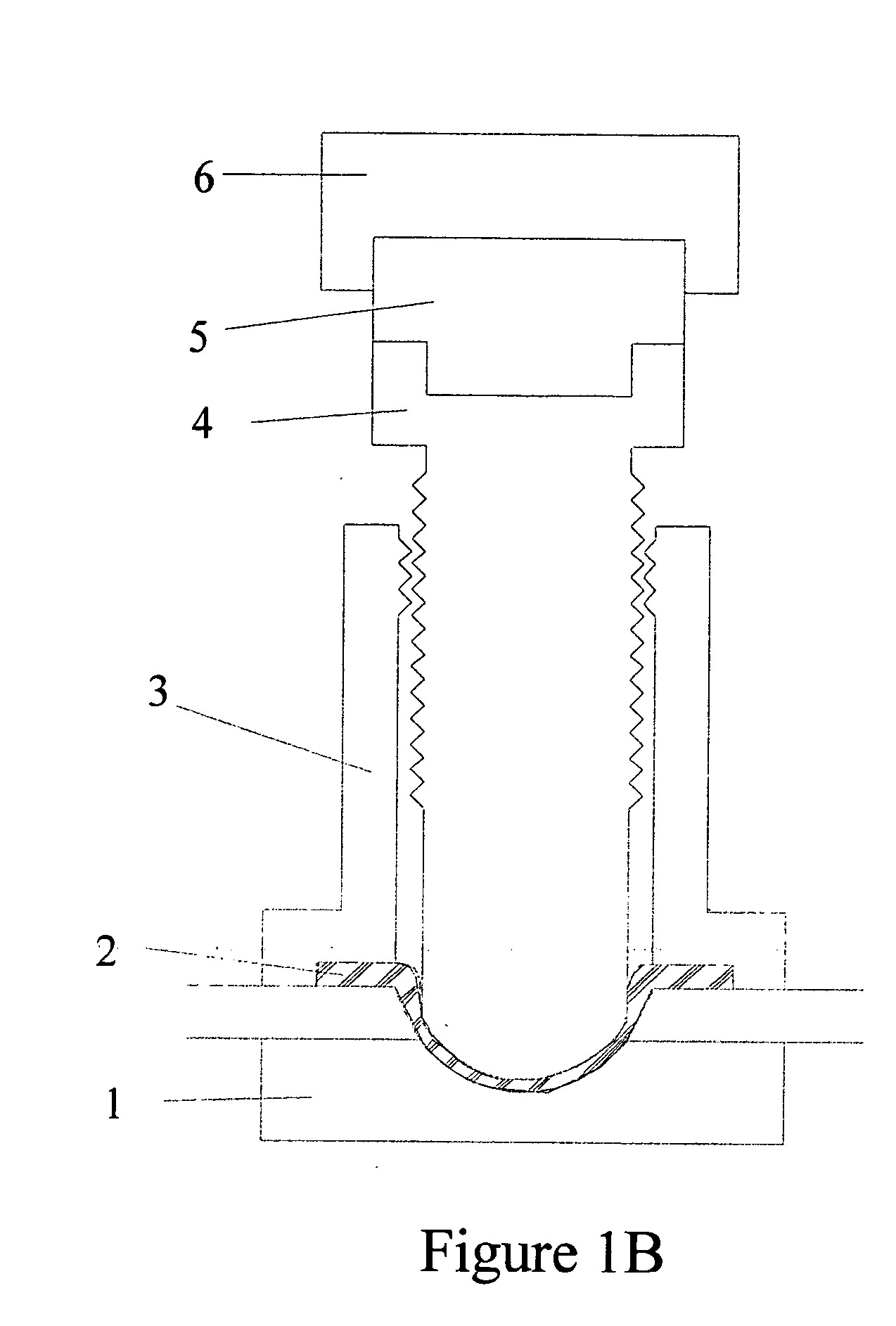
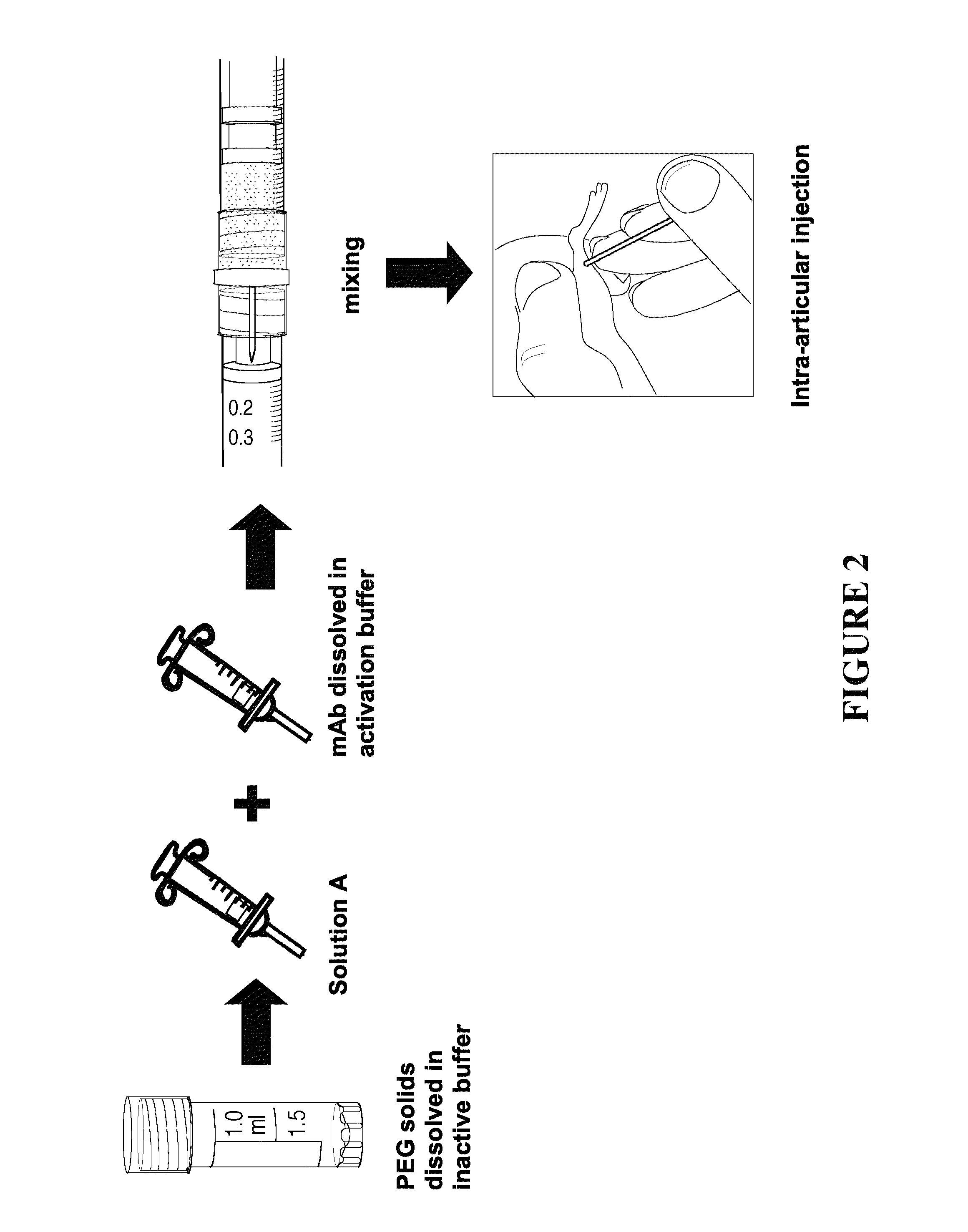
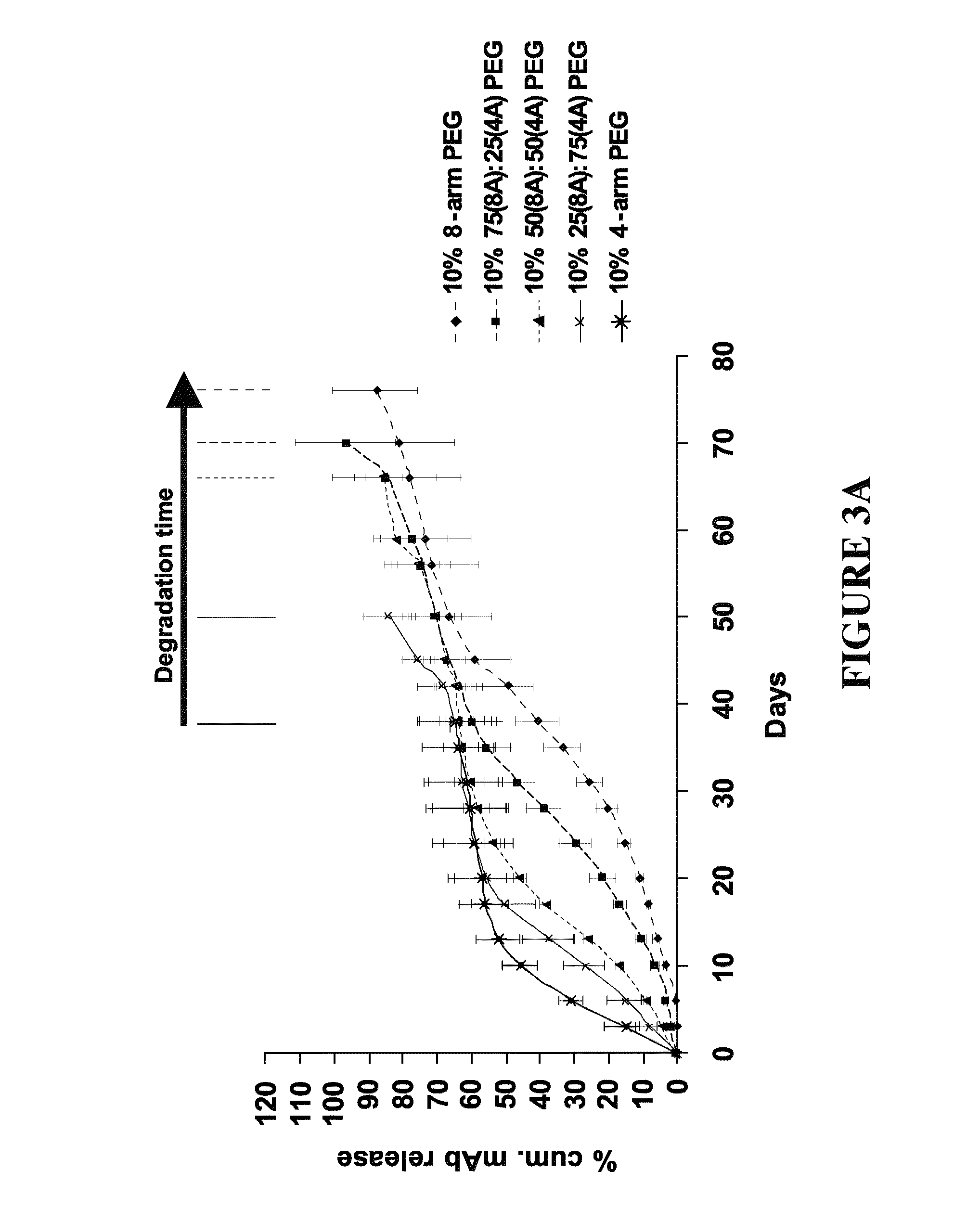
Delivery of biologic therapeutics
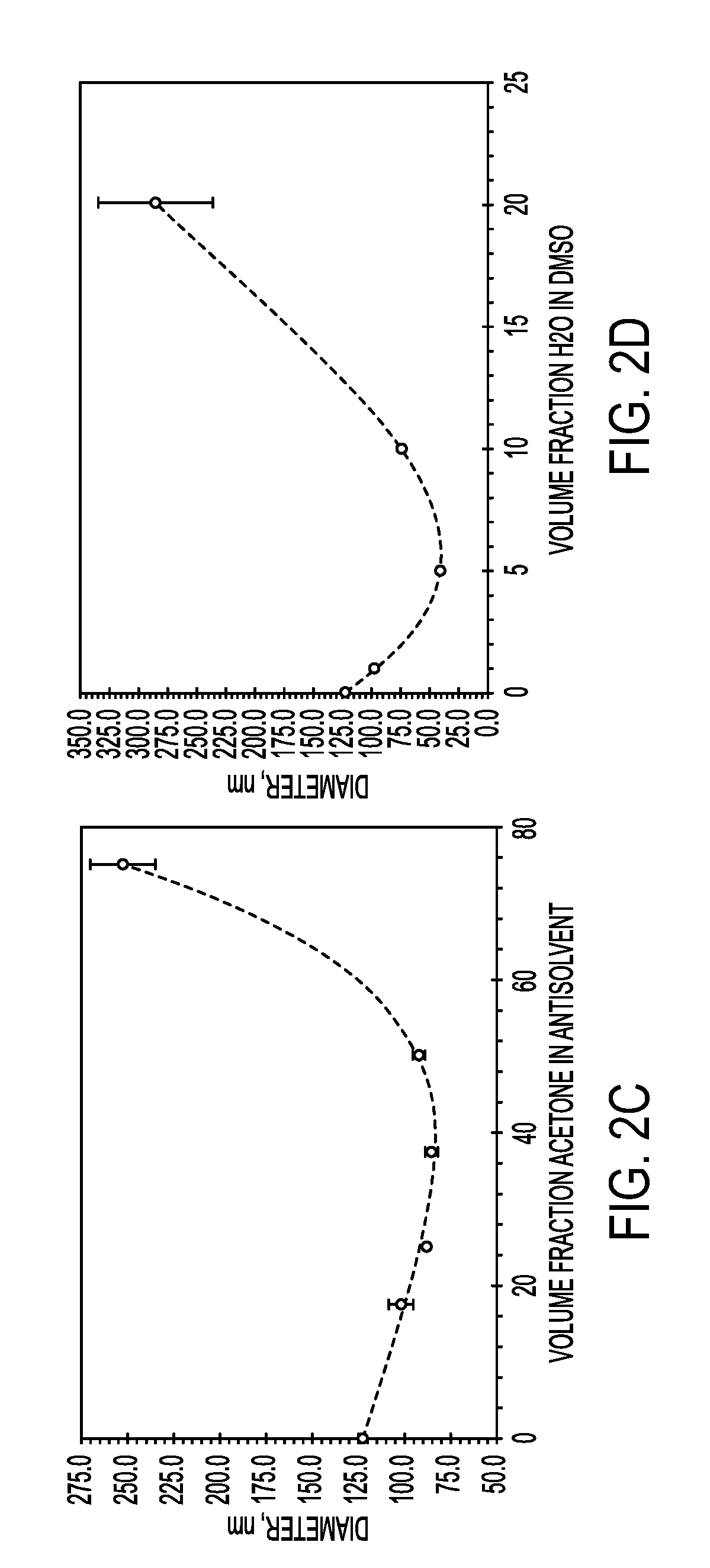
Formulations and methods are disclosed which provide controlled, sustained release of a biologic therapeutic to a space within the body. More specifically, formulations comprising a plurality of hydrophilic polymer strands, and methods of forming and administering such formulations, are disclosed. In some embodiments, the formulations exhibit a burst release, an initial release, a triphasic release, and release over thirty to ninety days of the biologic therapeutic. In some embodiments, the formulations exhibit reversible precipitation of the biologic therapeutic into precipitates having a diameter of about 50 nm to about 10 μm.
Owner:ABBOTT CARDIOVASCULAR
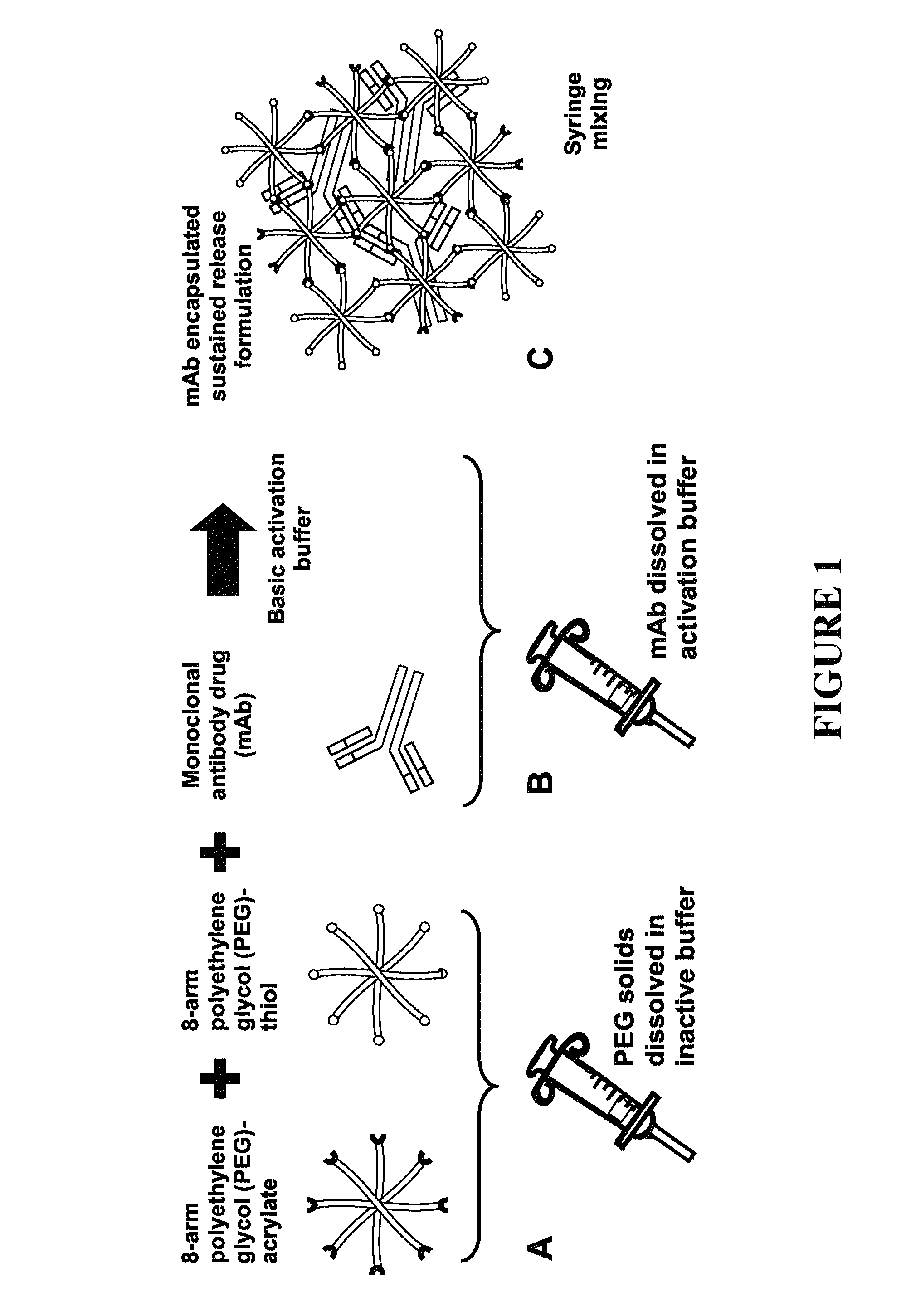
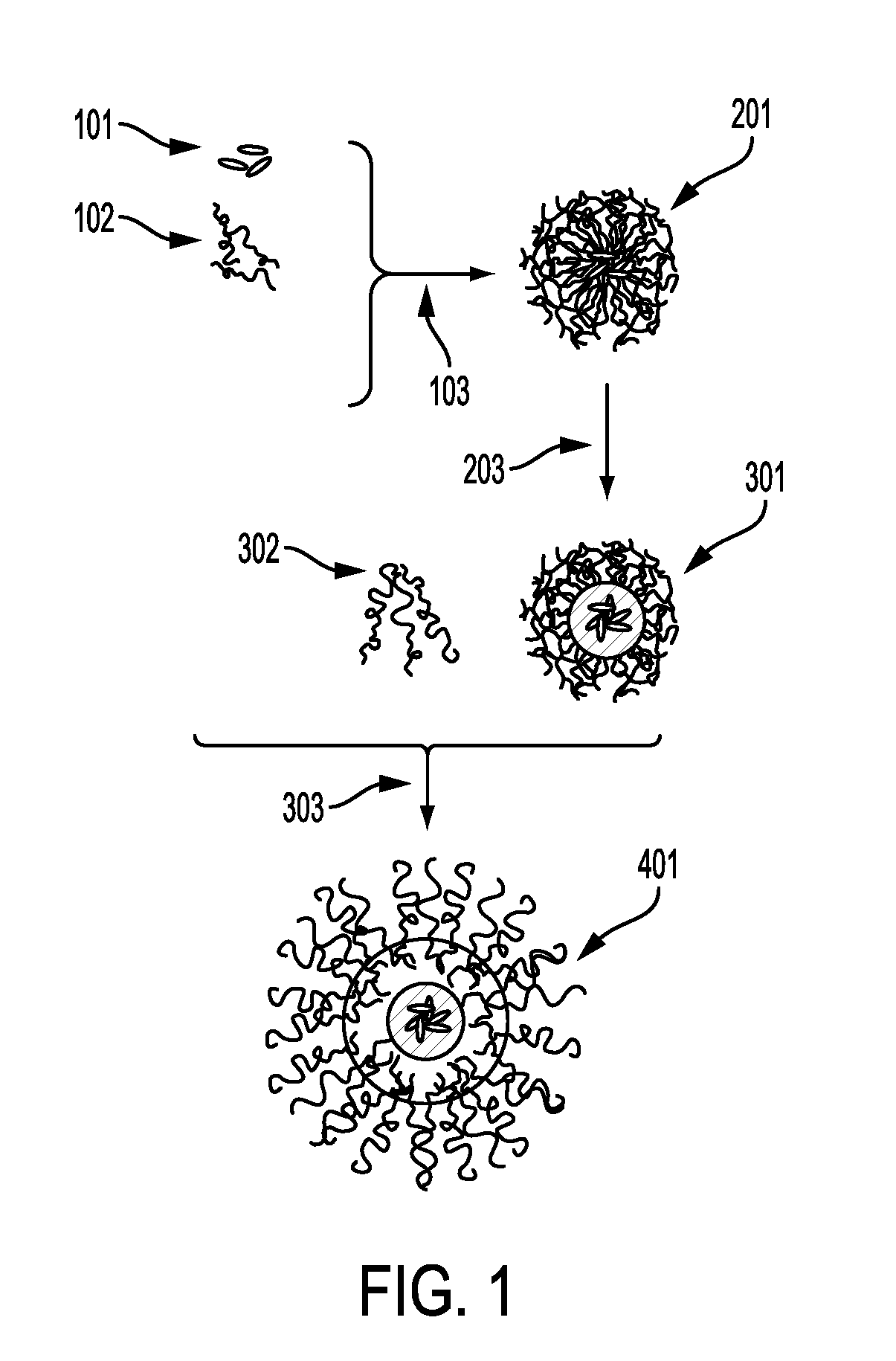
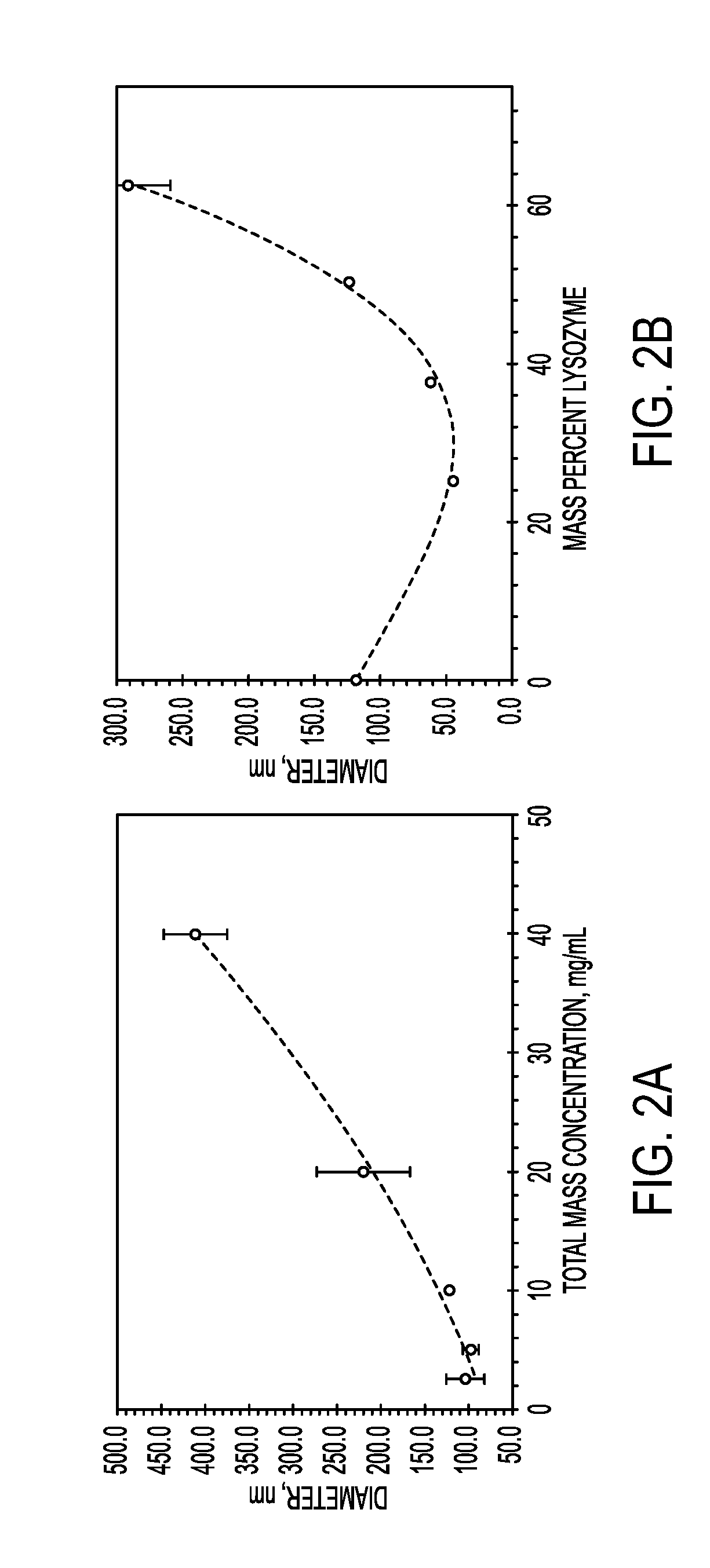
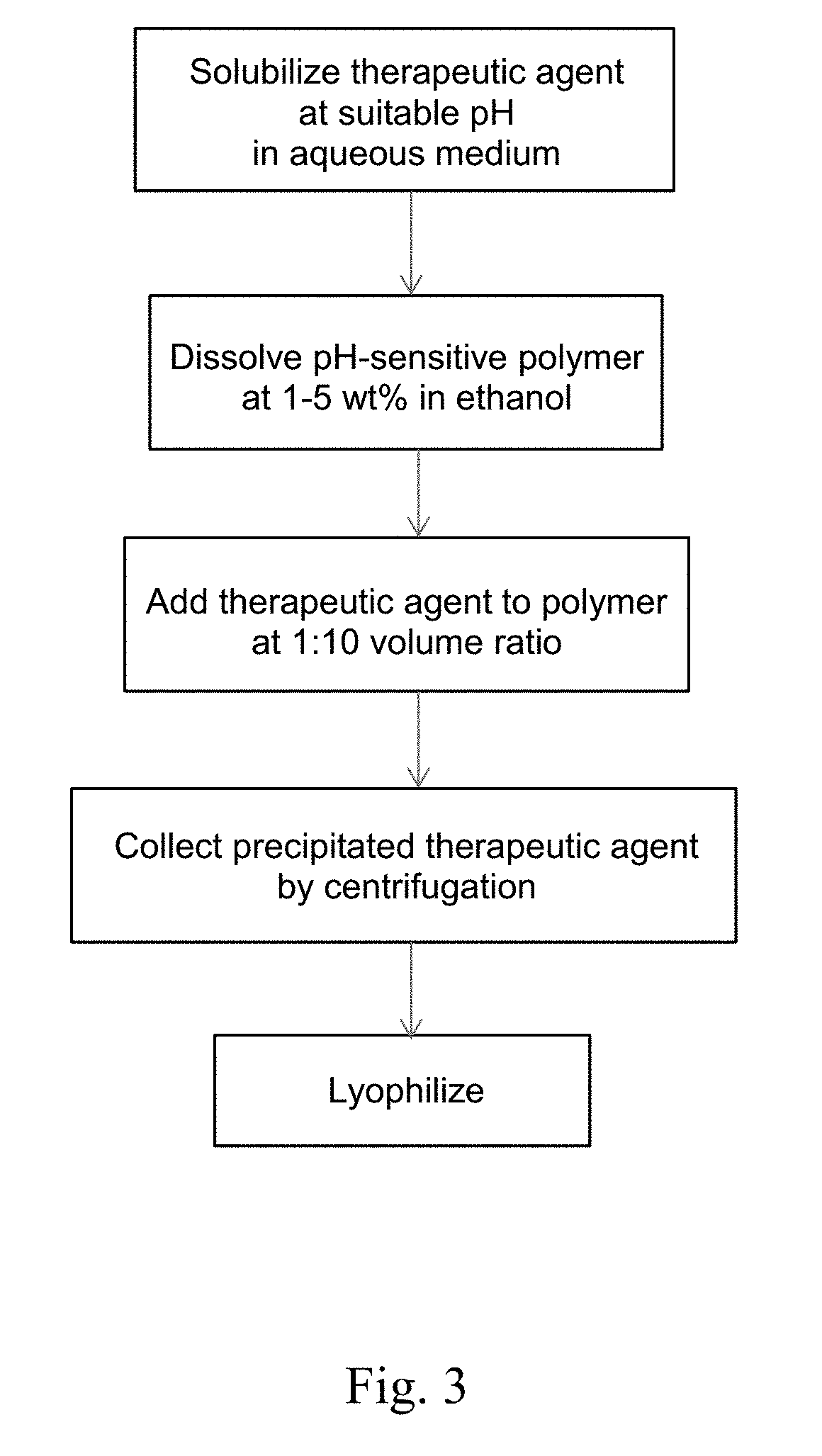
Process for encapsulating soluble biologics, therapeutics, and imaging agents
An “inverse” precipitation route to precipitate aqueous soluble species with copolymers as nanoparticles having a hydrophilic, polar core and a less polar shell is described. A method of the invention for encapsulating water soluble molecules using rapid, controlled precipitation is presented. Water soluble molecules—including peptides, proteins, DNA, RNA, non-biologic therapeutics, polysaccharide-based therapeutics (e.g., tobramycin) and imaging agents—precipitate into nano articles that are protected by a copolymer stabilizing agent. These particles may be covalently or non-covalently stabilized. The particles may be coated with an amphiphilic polymer, or processed into microparticles or larger monoliths. Post processing on the final construct may be conducted.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Bifidobacterium strain
ActiveUS20130004540A1Easy to mergeSimple preparation processBacteriaAntipyreticBifidobacteriumMicrobiology
Bifidobacterium strain AH121A is significantly immunomodulatory following oral consumption. The strain is useful as an immunomodulatory biotherapeutic agent.
Owner:MARS INC
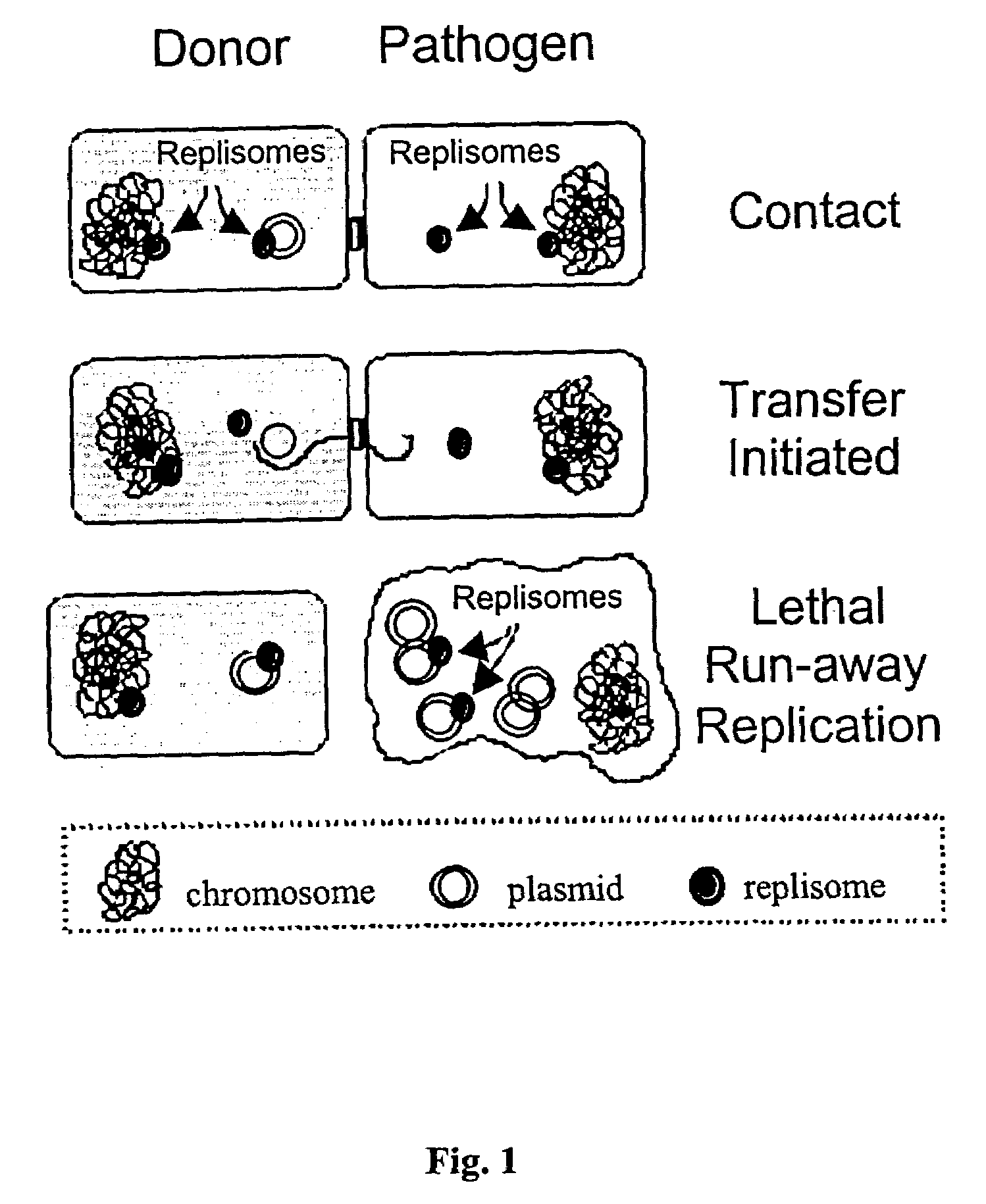
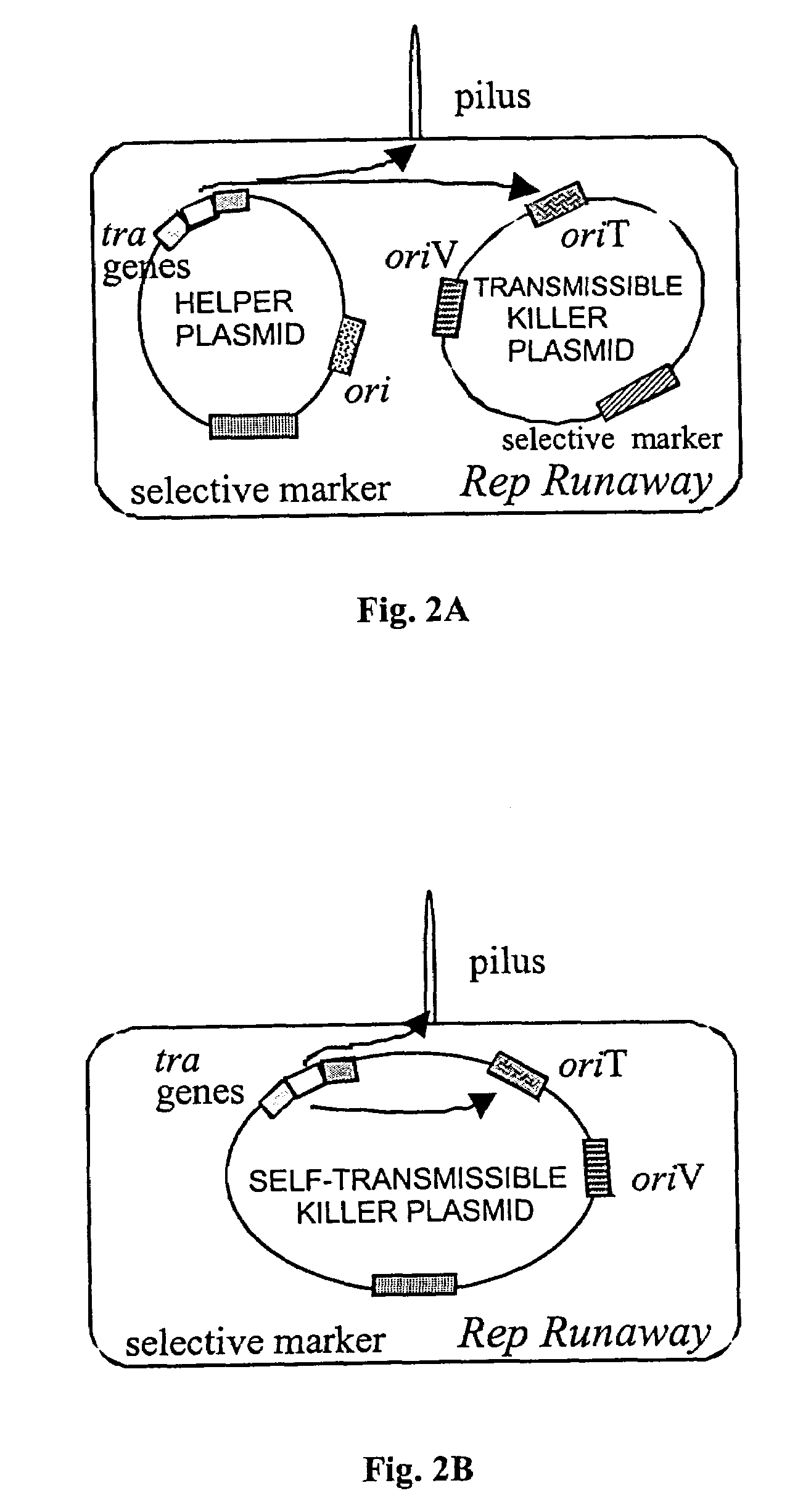
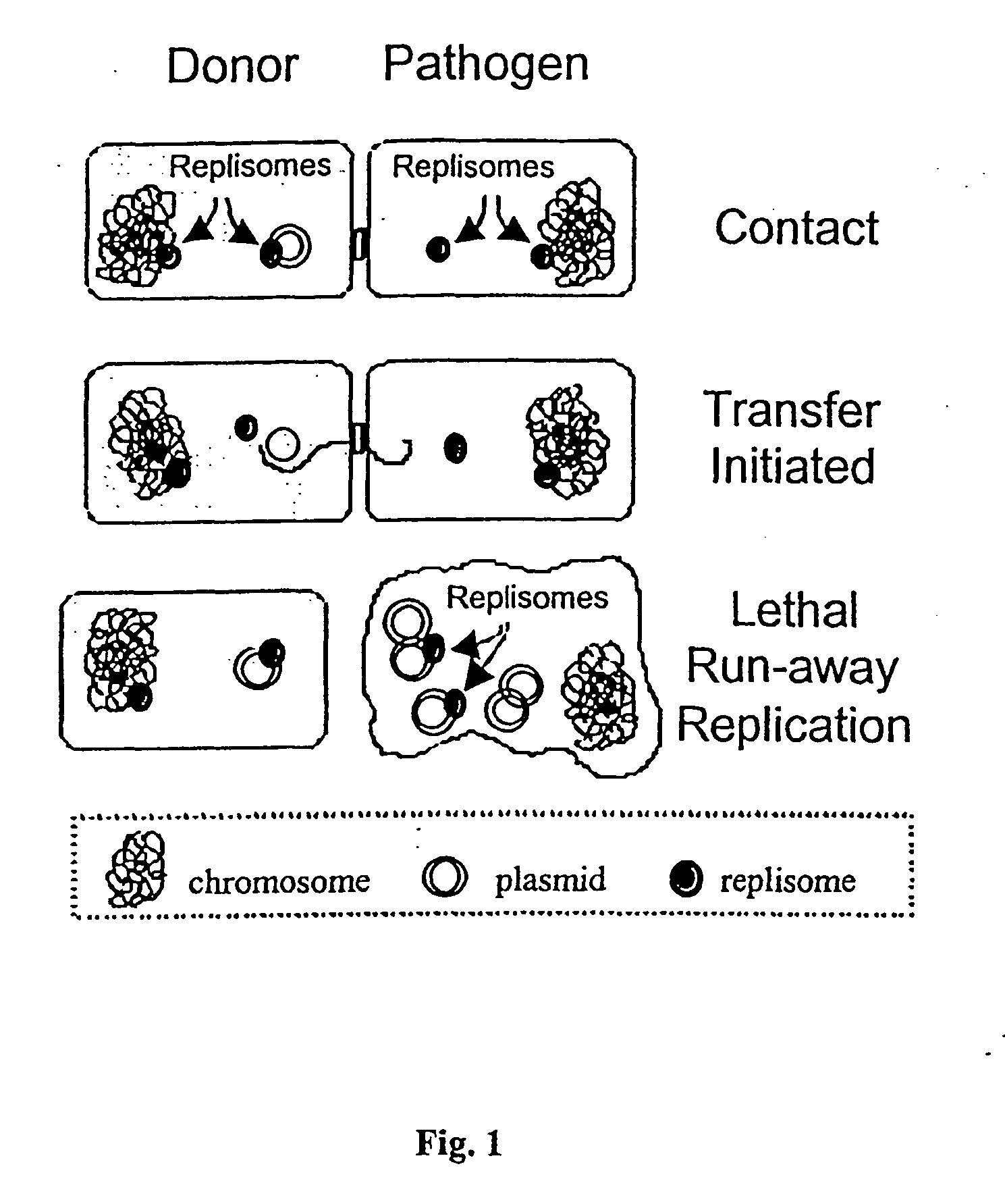
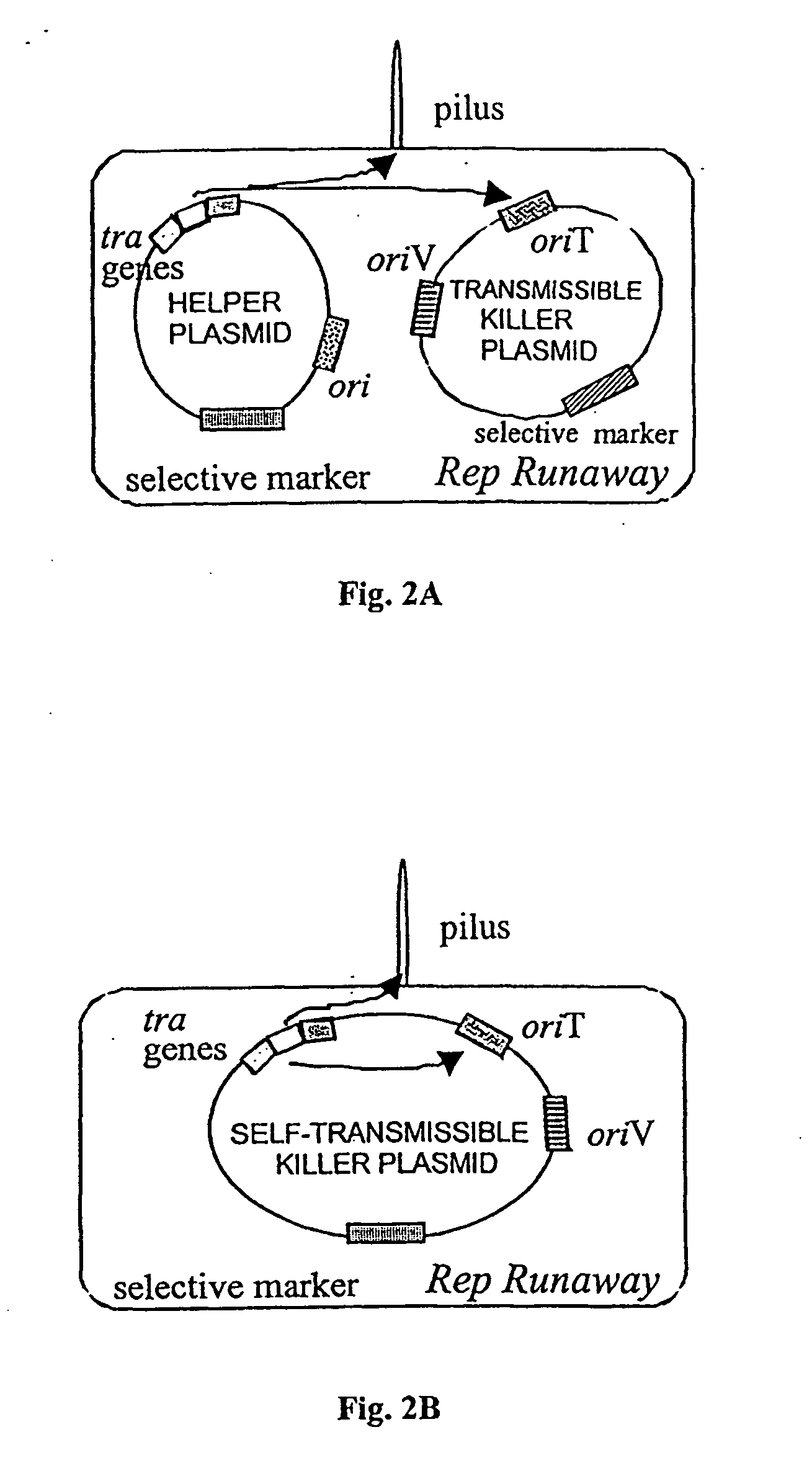
Anti-microbial biotherapeutic agents: alternatives to conventional pharmaceutical antibiotics
InactiveUS6991786B1Efficient transferFlexible rangeAntibacterial agentsBiocideBacteroidesMicrobial agent
Novel antimicrobial agents that can serve as replacements to conventional pharmaceutical antibiotics are disclosed. The antimicrobial agents comprise conjugatively transmissible plasmids that kill targeted pathogenic bacteria, but are not harmful to donor bacteria. Two types of lethal transmissible plasmids are disclosed. One type kills recipient bacteria by unchecked (“runaway”) replication in the recipient cells and is prevented from occurring in donor cells. Another type kills recipient bacteria by expressing a gene that produces a product detrimental or lethal to recipient bacterial cells, that gene being prevented from expression in donor cells.
Owner:WISCONSIN ALUMNI RES FOUND
LeY SPECIFIC BIOTHERAPEUTIC
InactiveUS20120010388A1Weak affinityGood curative effectImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationEpitopeBinding site
Embodiments are related to the field of immunology, and provide a highly avid LeY specific biotherapeutic including at least one further binding site having a different specificity to bind an epitope of a glycosylated cell surface molecule of a tumor cell, characterized by EC50 of less than 1 mM to confer immediate cytotoxicity to the tumor cell.
Owner:HIMMLER GOTTFRIED
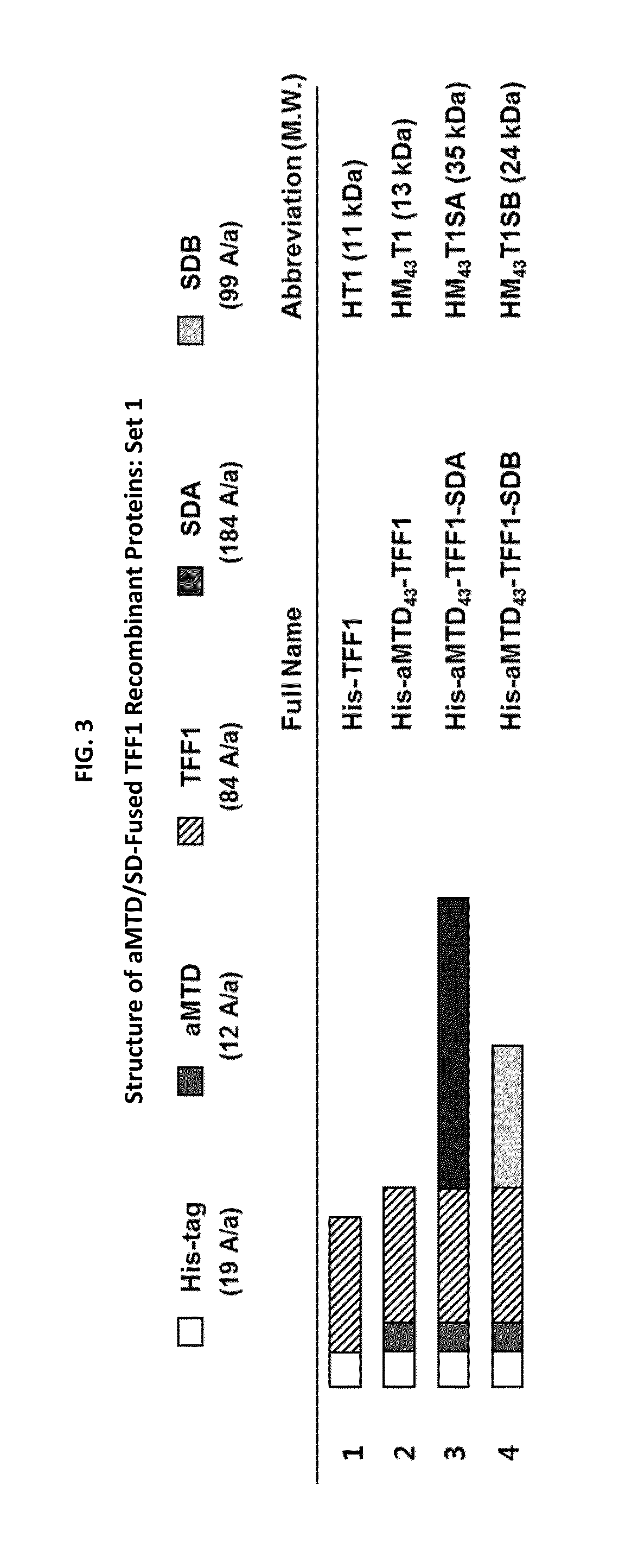
Development of Protein-Based Biotherapeutics That Penetrate Cell-Membrane and Induce Anti-Cancer Effect - Cell-Permeable Trefoil Factor 1 (CP-TFF1) in Gastrointestinal Track (GIT) Cancer, Polynucleotides Encoding The Same, and Anti-Cancer Compositions Comprising The Same
InactiveUS20160083441A1Improve efficiencyImprove solubilityPolypeptide with localisation/targeting motifSugar derivativesSolubilityNucleotide
The present study investigated the use of macromolecule intracellular transduction technology (MITT) to deliver biologically active TFF1 protein into gastric cancer cells both in vitro and in vivo. Proteins engineered to enter cancer cells are supposed to suppress cell proliferation and survival, consistent with its role as a tumor suppressor. The invention has developed new hydrophobic CPP-advanced MTDs (aMTDs) for high solubility / yield and cell- / tissue-permeability of the recombinant therapeutic fusion proteins. The TFF1 protein has been fused to aMTD165 and solubilization domains (SDs), and tested their therapeutic applicability as a gastric cancer-specific protein-based anti-cancer agent. Treatment with CP-TFF1 in gastric cancer cells reduced cancer cell viability (60%˜80% in 10 μM treatment), inhibited cell migration (approximately 50%). Furthermore, CP-TFF1 significantly inhibited the tumor growth during the treatment and the effect persisted for at least 3 weeks after the treatment was terminated (90% inhibition at day 42) in a xenografts model which were subcutaneously implanted with tumor block of gastric cancer cells (MKN45). In the present invention, CP-TFF1 recombinant protein showed the potential of novel protein therapies against gastric cancer.
Owner:JO DAEWOONG +1
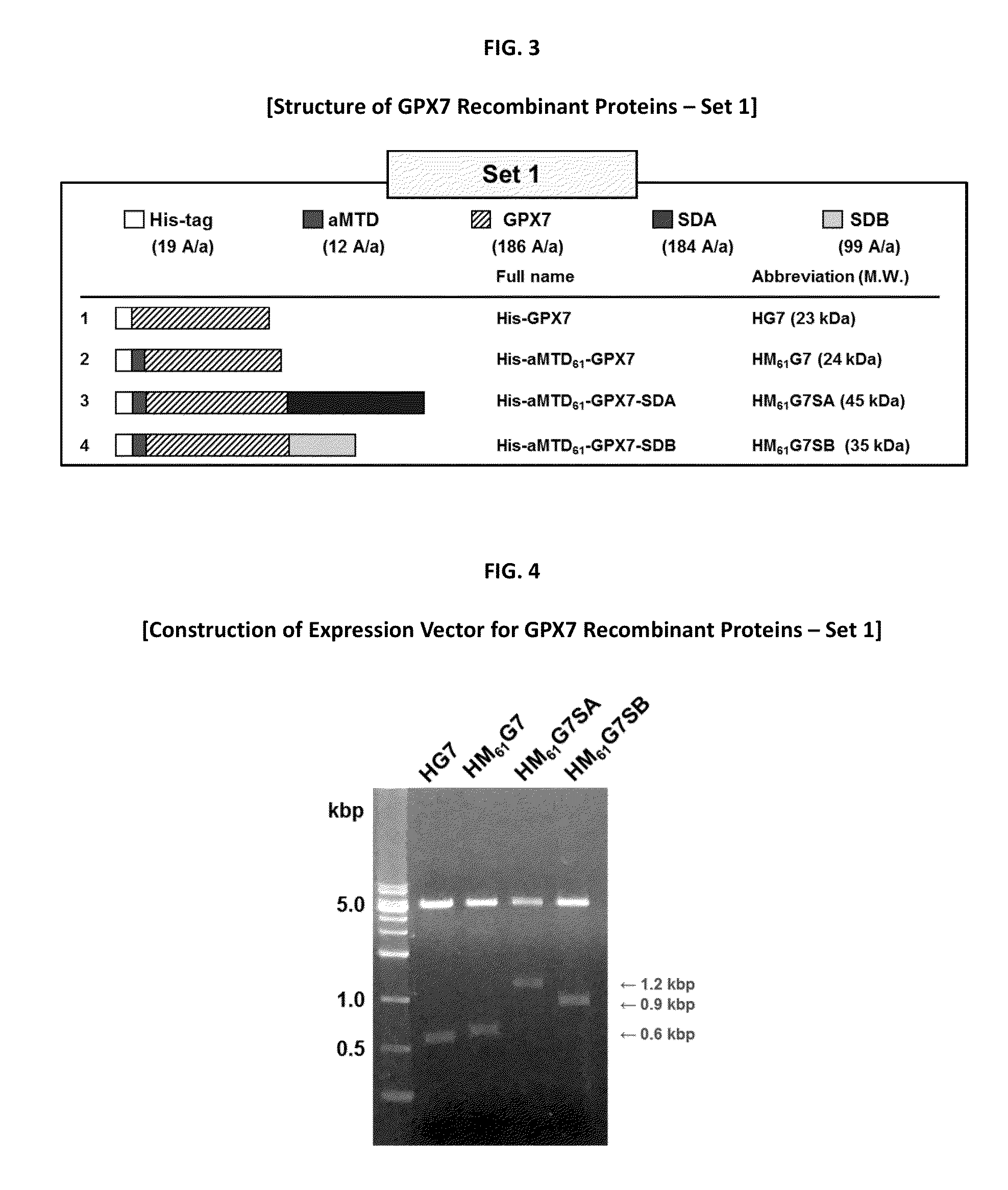
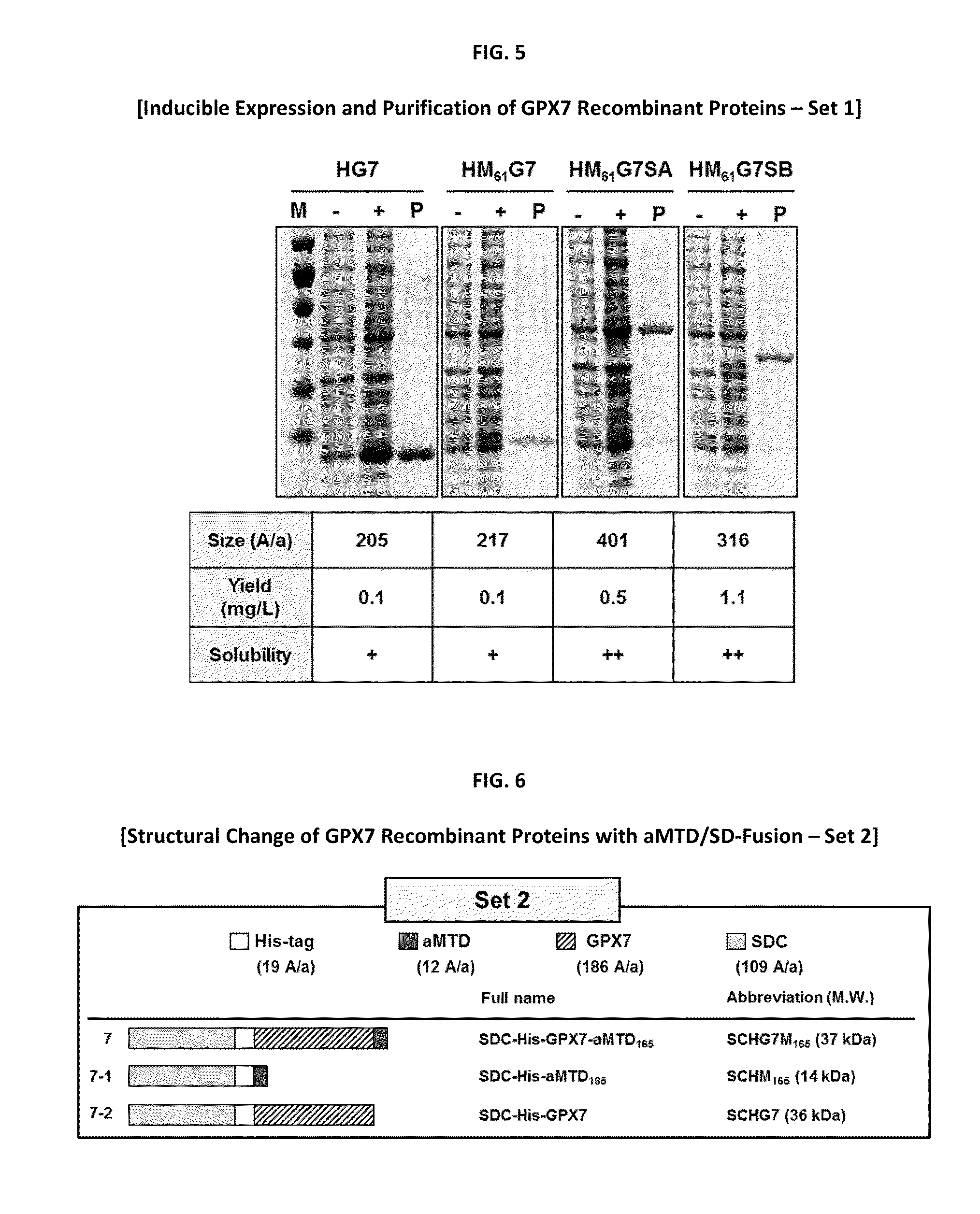
Development of Protein-Based Biotherapeutics That Penetrate Cell-Membrane and Induce Anti-Cancer Effect- Cell-Permeable Glutathione Peroxidase7 (CP-GPX7) in Gastrointestinal Track (GIT), Polynucleotides Encoding the Same, and Anti-Cancer Compositions Comprising the Same
InactiveUS20160068825A1Improve efficiencyImprove solubilityPolypeptide with localisation/targeting motifSugar derivativesCauses of cancerCell phenotype
Gastrointestinal track (GIT) including oesophageal and gastric cancers are a leading cause of cancer death worldwide. Limited therapeutic options highlight the need to understand the molecular changes responsible for the disease and to develop therapies based on this understanding. Advances in understanding the molecular changes responsible for GIT cancer etiology and progression are expected to improve disease diagnosis and treatment. The glutathione peroxidase 7 (GPX7) a candidate tumor suppressor implicated in GIT cancers including esophageal and gastric cancers has been implicated as a potential tumor suppressor gene in esophageal and gastric cancers; however, this claim is controversial. The goal of this invention is to develop cell-permeable (CP-) form of GPX7 to utilize the therapeutic potential of GPX7 in the treatment of GIT cancers. Using macromolecule intracellular transduction technology (MITT) enabled by novel hydrophobic cell-penetrating peptide (CPP) called advanced macromolecule transduction domains (aMTDs) which are able to promote protein uptake by mammalian cells and tissues, the first CP-GPX7 protein has been developed to deliver biologically active GPX7 protein into human oesophageal and gastric cancer cells, resulting in suppression of cell phenotypes and induction of changes in biomarker expression consistent with previously described effects of GPX7. CP-GPX7 recombinant protein fused to aMTD also suppresses the growth of human gastric tumors in a mouse xenograft model. The results of this art provide further evidence that GPX7 can function as an anti-cancer molecule and suggest that practical methods to augment GPX7 function could be useful in treating of some types of GIT cancers. The present art with CP-GPX7 recombinant protein illustrates the use of protein-based therapies to target GIT cancers.
Owner:CELLIVERY THERAPEUTICS +1
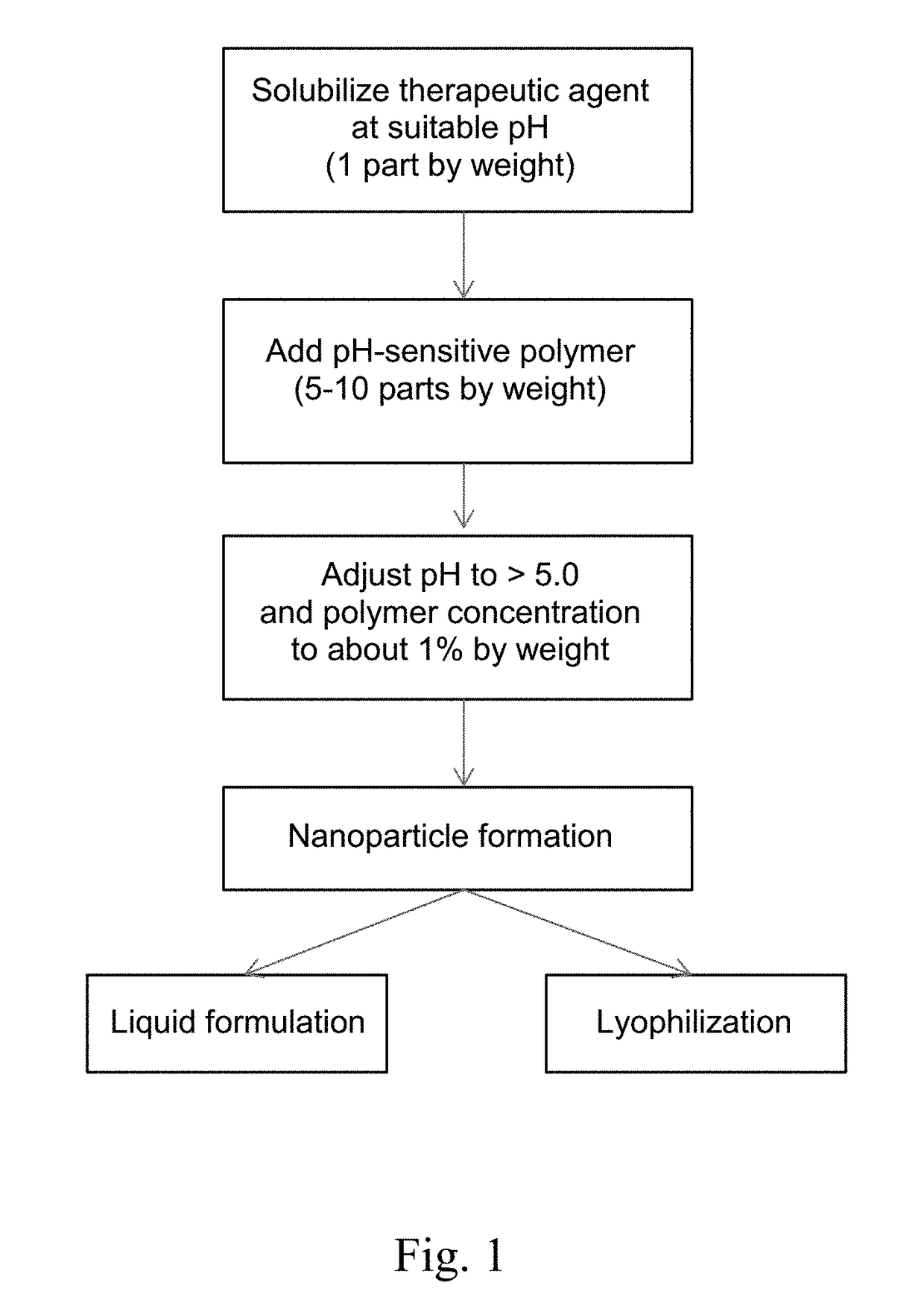
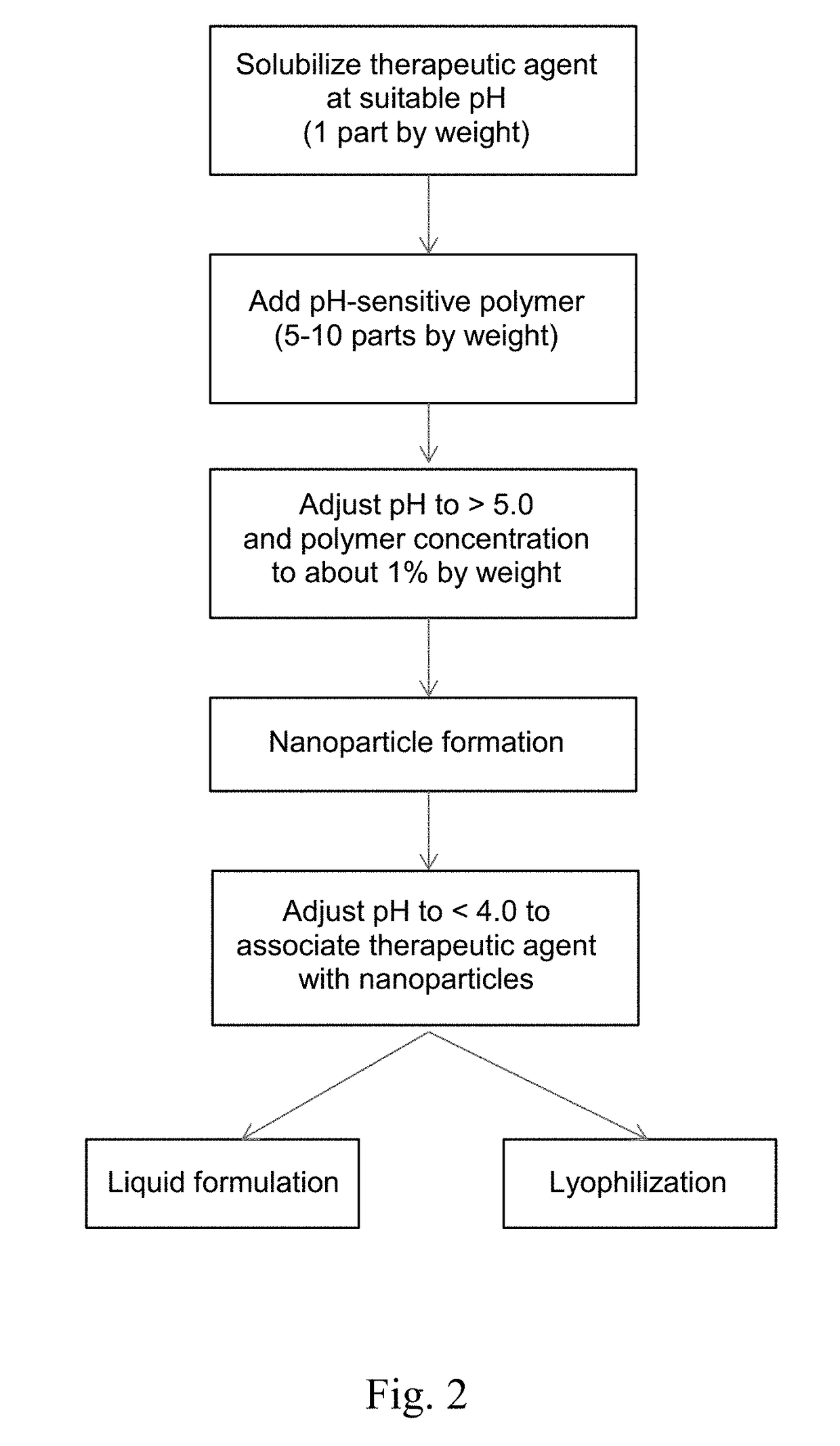
Formulations for Enteric Delivery of Therapeutic Agents
Formulations containing pH-sensitive nanoparticles for the enteric delivery of therapeutic agents are provided. The nanoparticles include a pH-sensitive polymer that protects the therapeutic agent against degradation in the stomach and allows it to be released in the small intestine or colon. The nanoparticle formulation is particularly effective at protecting sensitive biotherapeutic agents from degradation when administered orally, and makes it possible to avoid administration of such agents by injection. Also provided are methods for producing the formulations, as well as methods of treating diseases employing the formulations.
Owner:CURIRX INC
Immune Effector Cells Pre-Infected with Oncolytic Virus
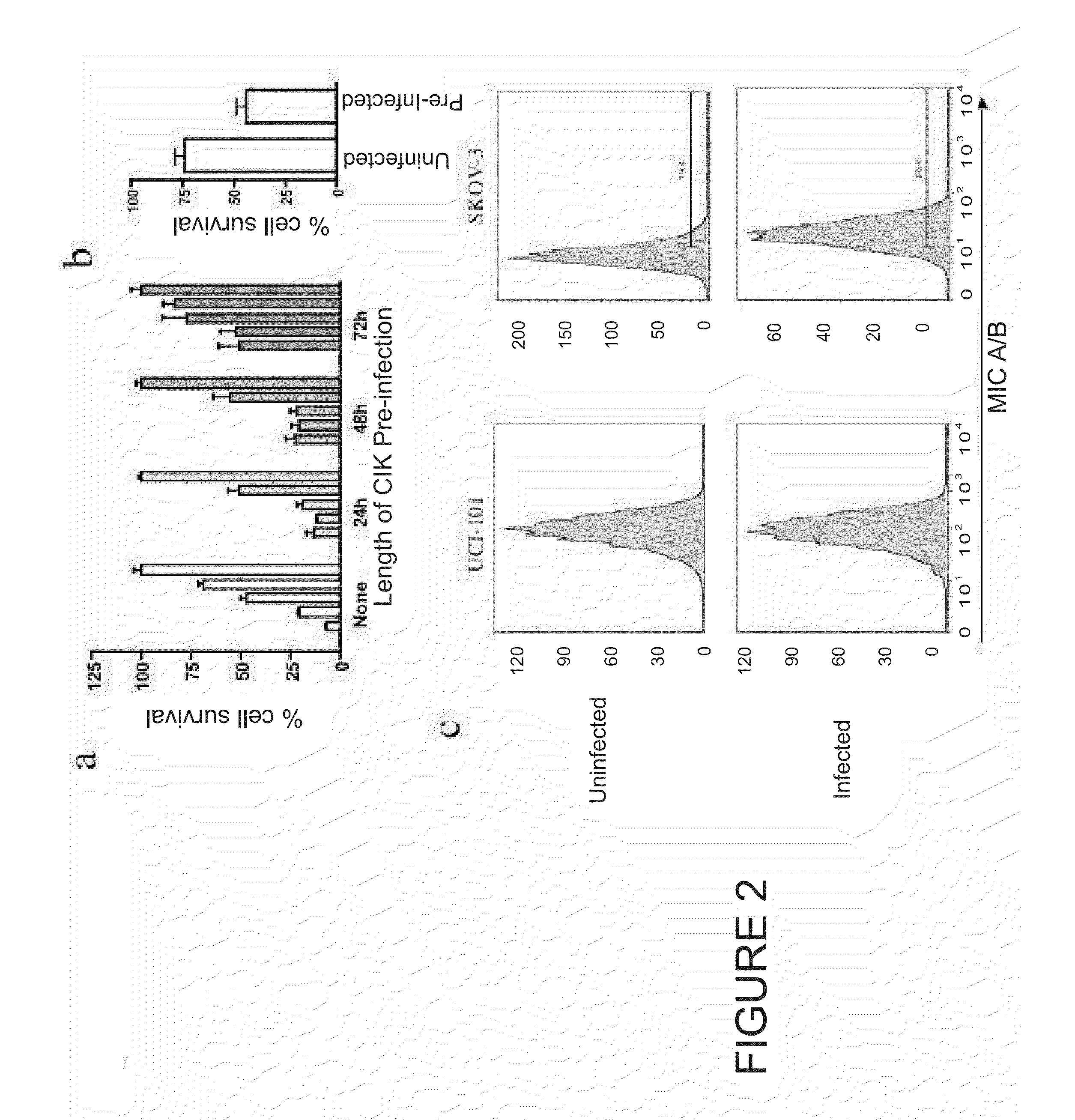
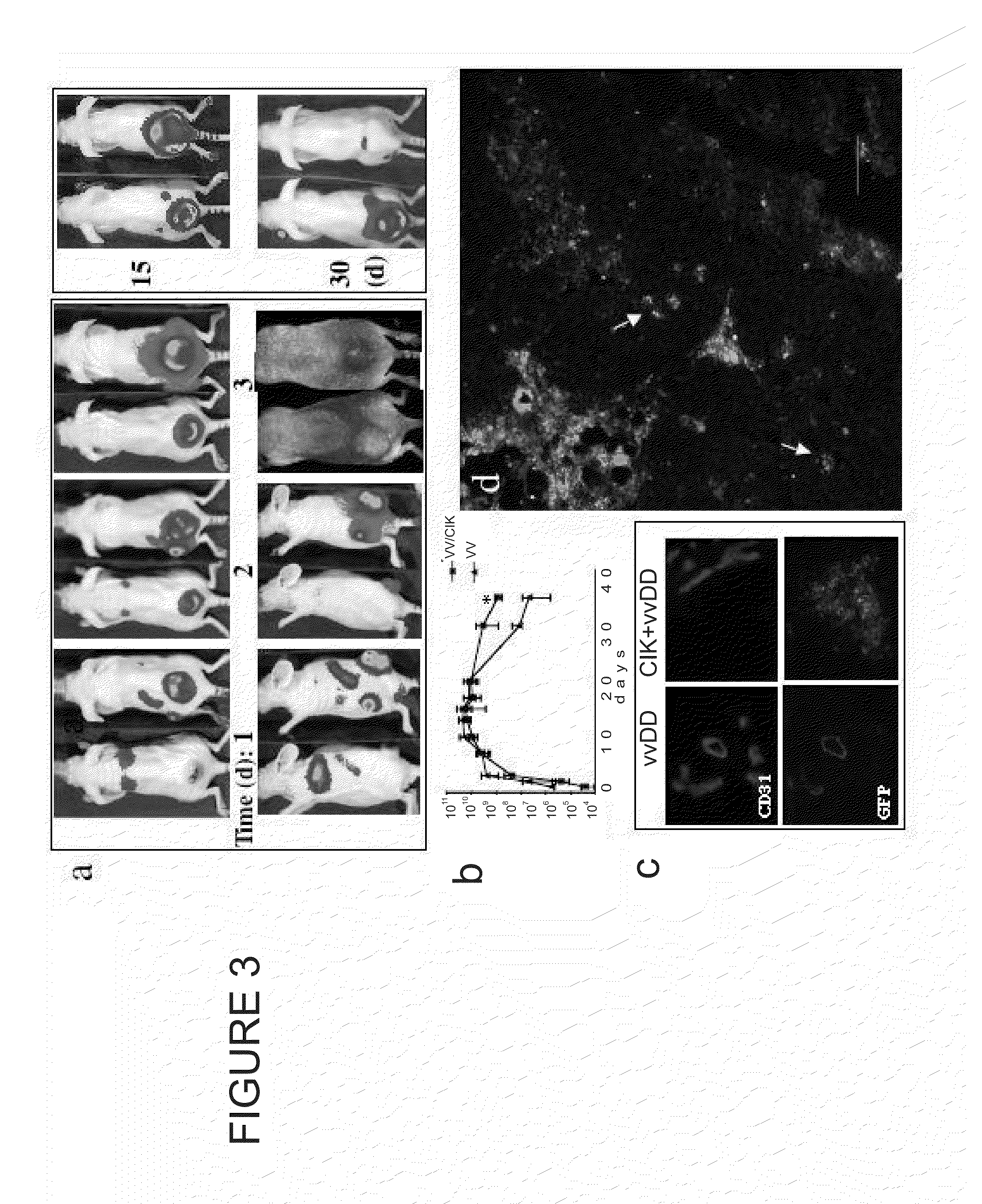
Compositions and methods are provided for the treatment of cancer. An immune effector cell population is pre-infected with an oncolytic virus. The combined therapeutic is safe and highly effective, producing an enhanced anti-tumor effect compared to either therapy alone. The methods of the invention thus provide for a synergistic effect based on the combined biotherapeutics.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
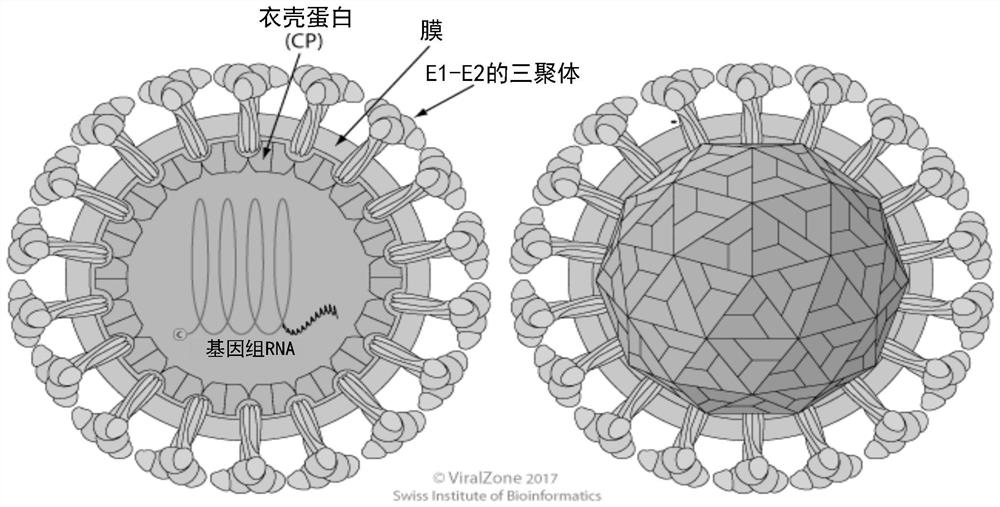
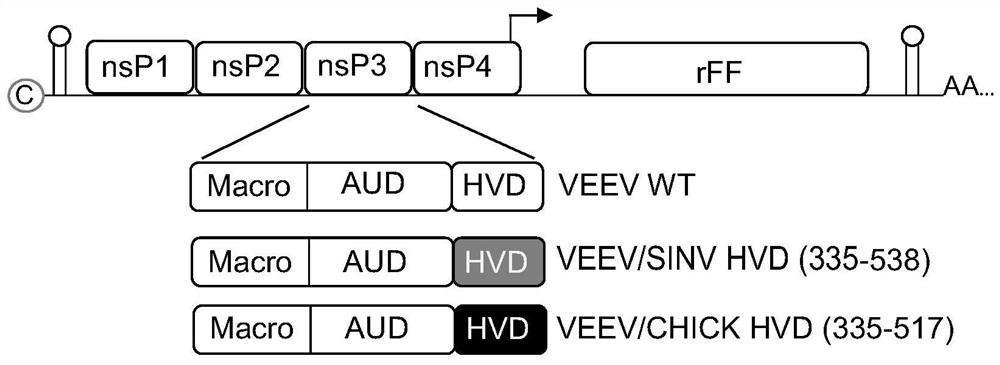
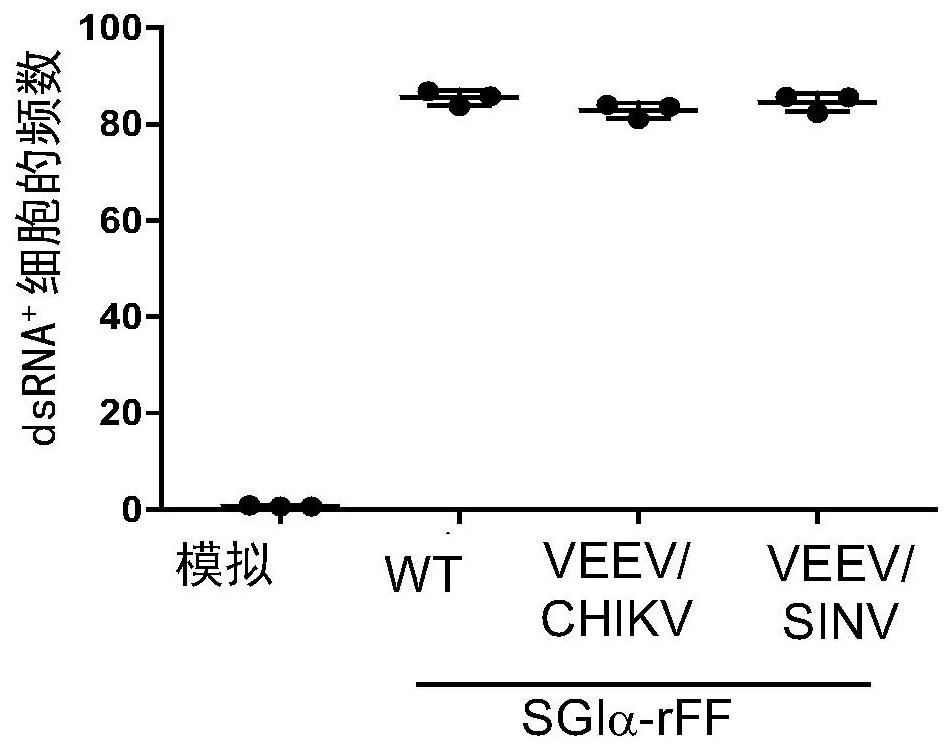
Alphavirus-based replicons for administration of biotherapeutics
PendingCN113227122ASsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousStructural protein
The invention provides RNA replicons useful for administering a heterologous protein or peptide into a mammal and eliciting a reduced immune response or no immune response from the mammal. The RNA replicons have RNA sequences encoding for a heterologous protein or peptide, New World alphavirus nonstructural proteins nsP1, nsP2, and nsP4; and an alphavirus nsP3 protein macro domain, central domain, and hypervariable domain. The encoded hypervariable domain can have an amino acid sequence derived from an Old World alphavirus nsP3 hypervariable domain; or can have an amino acid sequence derived from a portion of a New World alphavirus nsP3 hypervariable domain, and another portion derived from an Old World alphavirus nsP3 hypervariable domain.
Owner:JANSSEN PHARMA NV
Anti-microbial biotherapeutic agents: alternatives to conventional pharmaceutical antibiotics
InactiveUS20050163757A1Efficient transferEffective alternativeAntibacterial agentsBiocideBacteroidesMicrobial agent
Novel antimicrobial agents that can serve as replacements to conventional pharmaceutical antibiotics are disclosed. The antimicrobial agents comprise conjugatively transmissible plasmids that kill targeted pathogenic bacteria, but are not harmful to donor bacteria. Two types of lethal transmissible plasmids are disclosed. One type kills recipient bacteria by unchecked (“runaway”) replication in the recipient cells and is prevented from occurring in donor cells. Another type kills recipient bacteria by expressing a gene that produces a product detrimental or lethal to recipient bacterial cells, that gene being prevented from expression in donor cells.
Owner:FILUTOWICZ MARCIN S
Application of Lactobacillus paracasei to CAR (chimeric antigen receptor)-T cell therapy
ActiveCN110305821AStrong immunomodulatory activityEnhance immune functionAntibacterial agentsBacteriaMicroorganismTumor therapy
The invention relates to an application of Lactobacillus paracasei to CAR (chimeric antigen receptor)-T cell therapy, belongs to the technical field of microorganisms and particularly discloses Lactobacillus paracasei MIT-16 and an application of the Lactobacillus paracasei to the immunological therapy process of canine tumors. The Lactobacillus paracasei MIT-16 with higher acid-producing abilityis separated and screened from an intestinal tract of a mouse, has good acid resistance, stronger bactericidal capacity and remarkable immune regulation functions, particularly plays an important rolein immune response regulation in tumor therapy and can be used as an immune regulation biotherapy agent.
Owner:LUDONG UNIVERSITY
High potency stable formulations of vaginal Lactobacillus
This invention provides for a dry preserved formulation of Lactobacillus suitable for administration to people as a pro-biotic or live biotherapeutic treatment where the formulation is stable, has high potency, and contains no animal-derived excipients.
Owner:OSEL
Systems and Methods for Identifying Protein Aggregates in Biotherapeutics
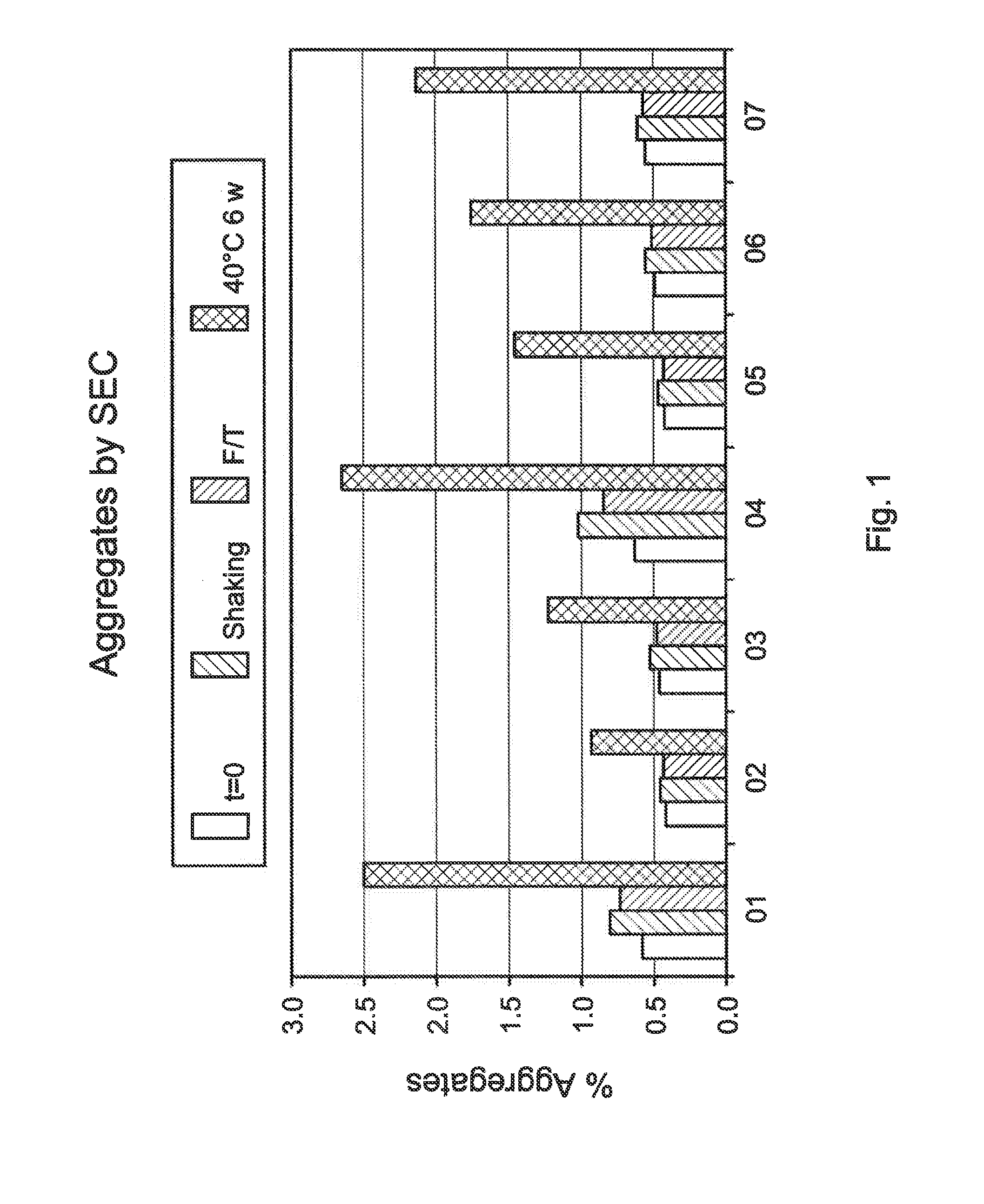
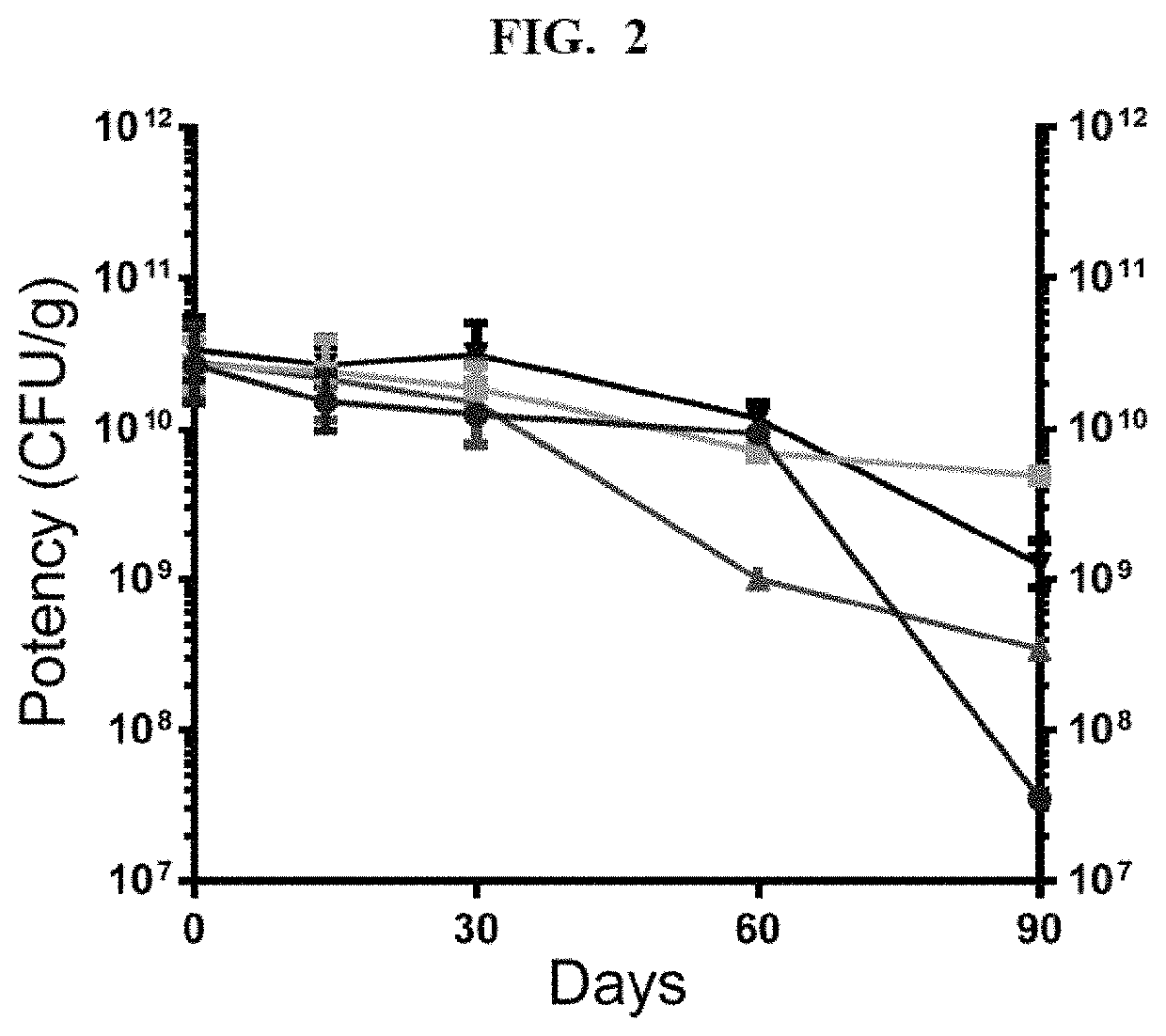
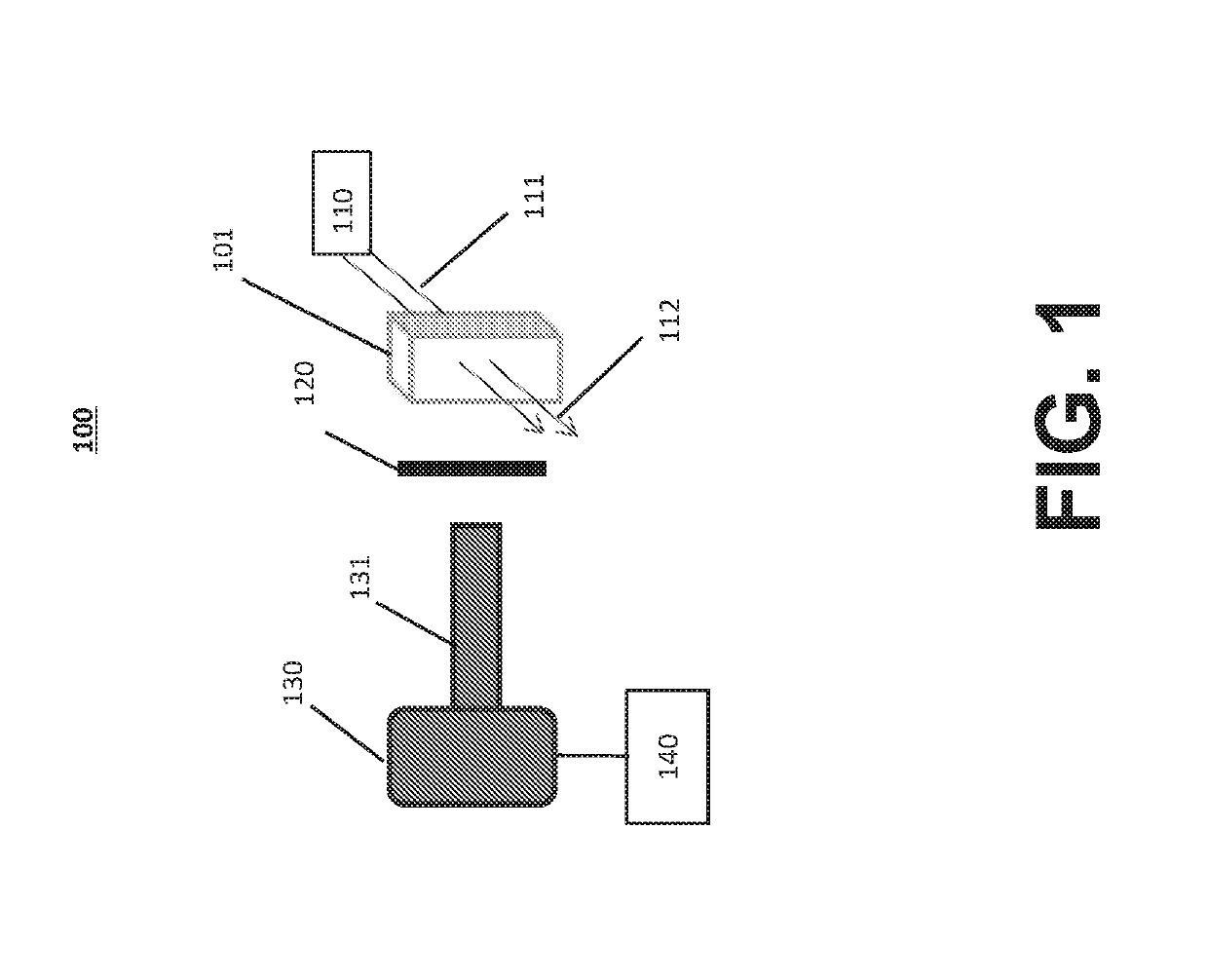
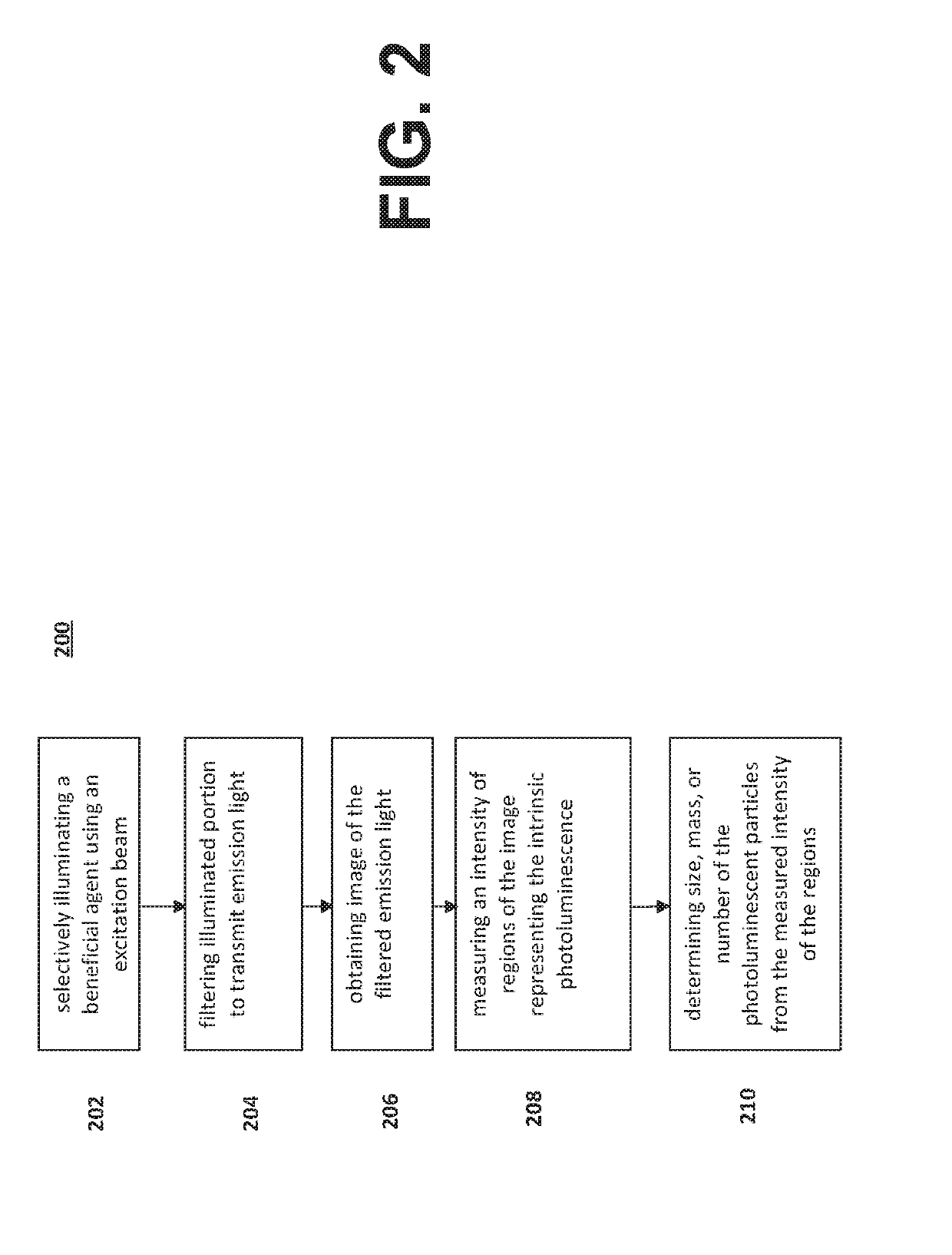
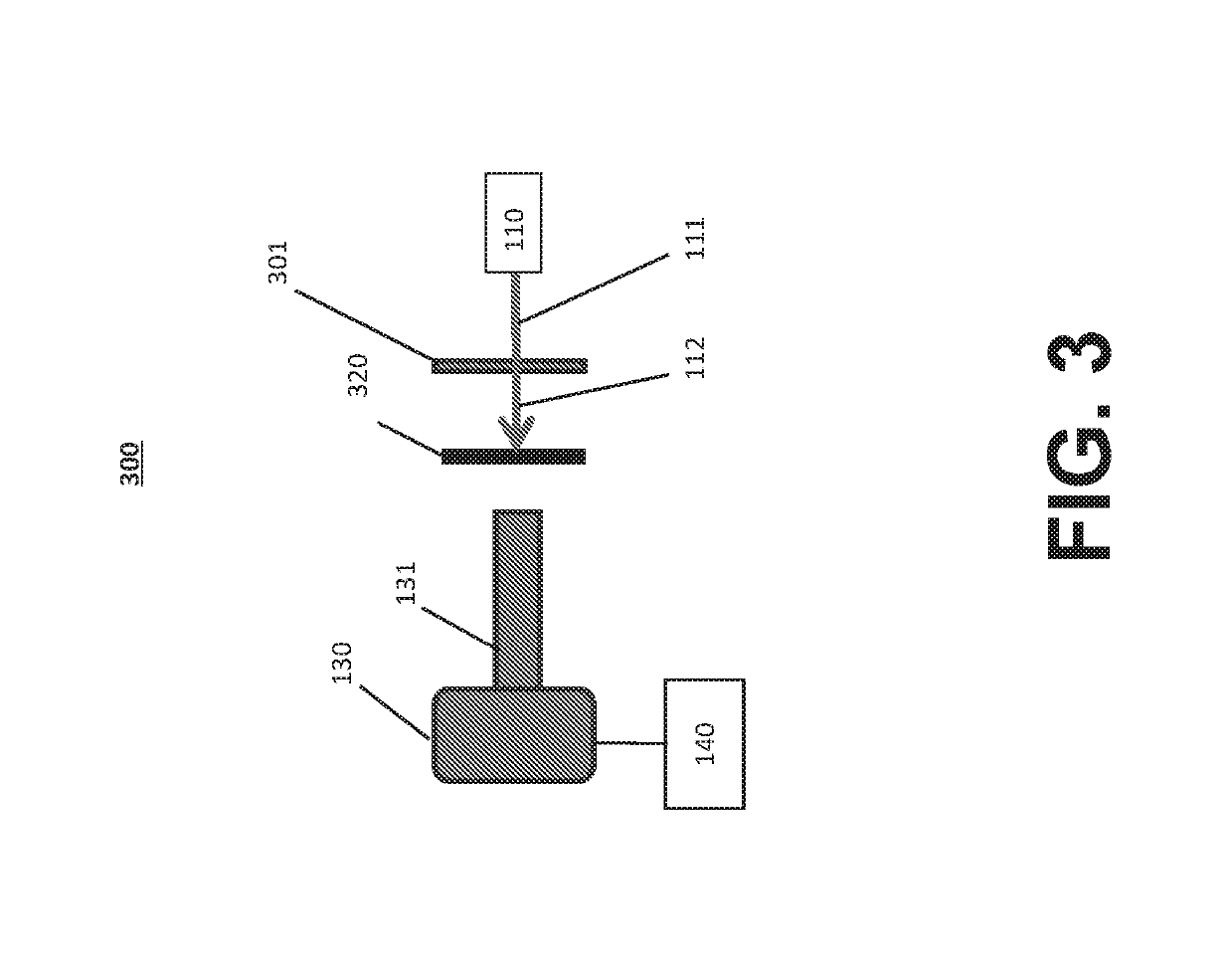
Systems and methods for inspecting particles in a liquid beneficial agent are provided. Inspecting particles in a liquid beneficial agent includes selectively illuminating at least a portion of a liquid beneficial agent contained within a container using an excitation beam configured to excite photoluminescent particles in the liquid beneficial agent to emit an emission light and produce scattered excitation light, filtering the illuminated portion of the liquid beneficial agent to transmit the emission light and block the scattered excitation light, obtaining an image of the filtered emission light, analyzing image data representing the image of the filtered emission light to detect regions of the image representing the intrinsic photoluminescence of the photoluminescent particles, measuring an intensity of the regions of the image representing the intrinsic photoluminescence of the photoluminescent particles, and determining a size or number of the photoluminescent particles from the measured intensity of the regions.
Owner:ABBVIE INC
Anti-tumor polypeptide HUSP-48 and application thereof
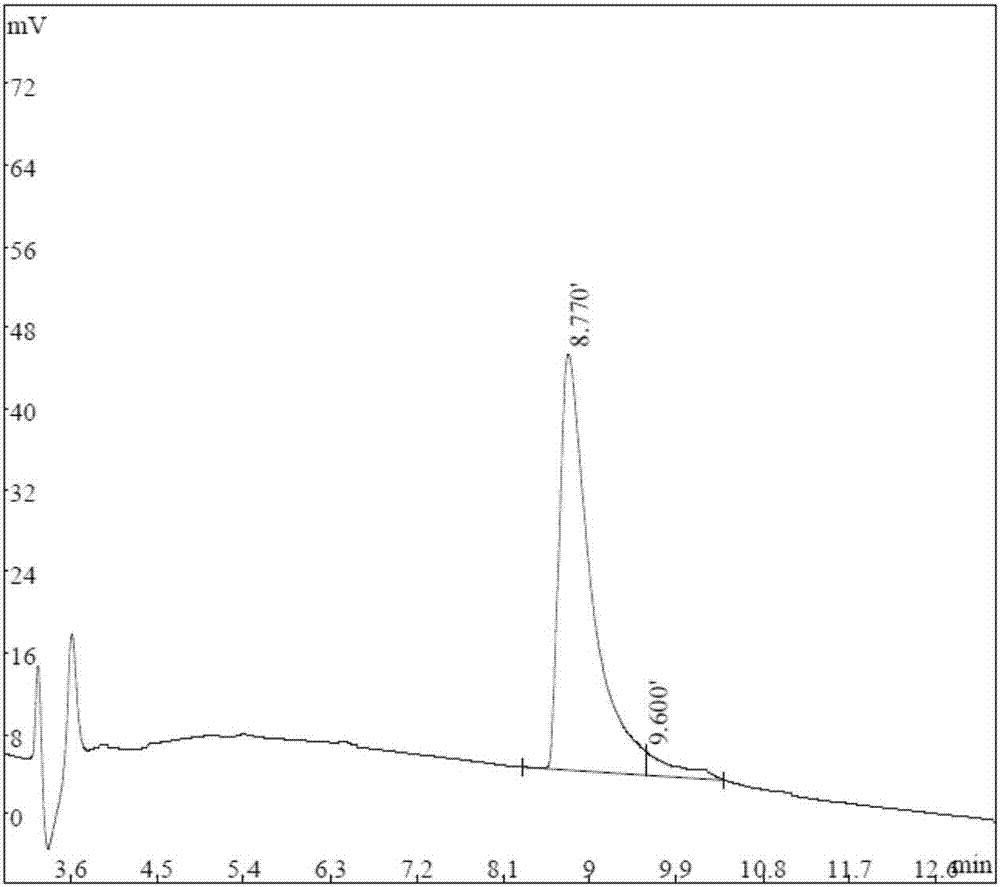
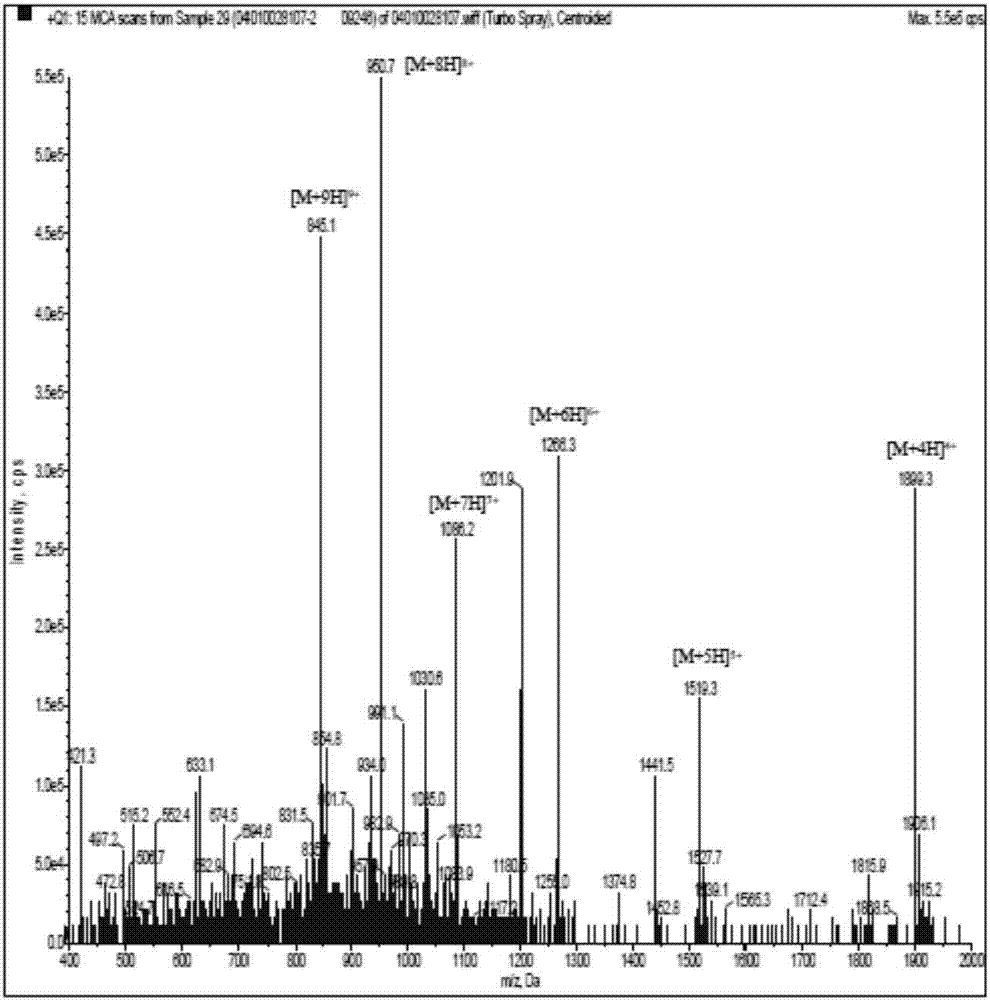
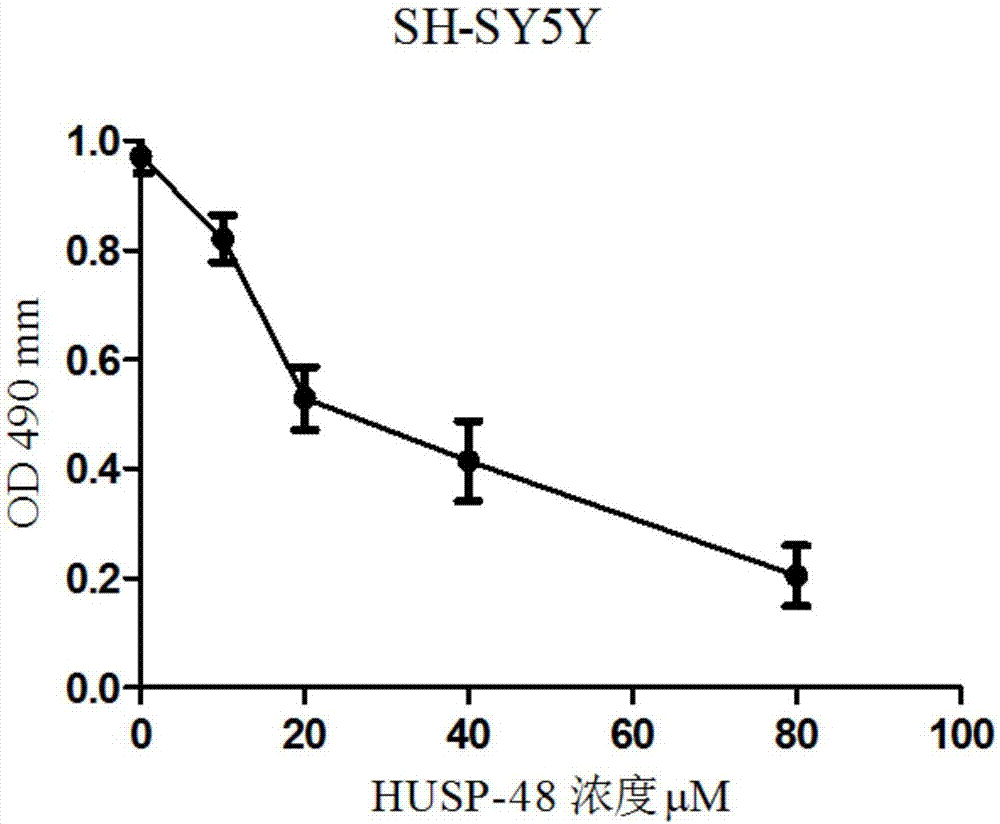
ActiveCN107082799ATumor suppressionInhibition of migratory invasion effectsPolypeptide with localisation/targeting motifPeptide-nucleic acidsCytotoxicityBiotherapeutic agent
The present invention provides a polypeptide capable of killing tumor cells and application thereof. The amino acid sequence of the polypeptide is as shown in SEQ ID NO: 1. The invention also relates to an anti-tumor polypeptide and application thereof. The anti-tumor polypeptide comprises a tumor cell killing structural domain and a transmembrane structural domain. The amino acid sequence of the transmembrane structural domain is as shown in SEQ ID NO: 2. The transmembrane structural domain of the anti-tumor polypeptide has no cytotoxicity, but has an obvious effect of inhibiting tumor proliferation and migration invasion after connected to the tumor cell killing structural domain. The anti-tumor polypeptides can be individually used as an anti-tumor biotherapeutic agent and also can inhibit tumors in combination with other treatment methods.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Methods of treating cancer
ActiveUS20190269711A1Avoid inductionSsRNA viruses positive-sensePeptide/protein ingredientsCaspase inhibitorsControl release
The present invention relates to methods of treating cancer in a human subject in need thereof. In particular, the present invention relates to treating a cancer by administering a recombinant virus which expresses one or more biotherapeutic agents in a subject, and administering to the subject a nucleotide analogue or nucleotide precursor analogue chemotherapeutic agent. The invention further relates to method for treating cancer by administering a nucleotide analogue or nucleotide precursor analogue chemotherapeutic agent and a caspase inhibitor, and, optionally, also administering a recombinant virus expressing one or more biotherapeutic agents in the subject. The invention also relates to a method for treating cancer by administering purified interferon gamma to a subject and administering to the subject a nucleotide analogue or nucleotide precursor analogue chemotherapeutic agent. Also provided are pharmaceutical compositions, including controlled release pharmaceutical compositions containing: a nucleotide analogue or nucleotide precursor analogue chemotherapeutic agent and a recombinant virus; a nucleotide analogue or nucleotide analogue precursor chemotherapeutic agent and a caspase inhibitor; or a purified interferon gamma and a nucleotide analogue or nucleotide precursor analogue chemotherapeutic agent.
Owner:ASCEND BIOPHARMACEUTICALS PTY LTD
Microbiome-based informed method to formulate live biotherapeutics
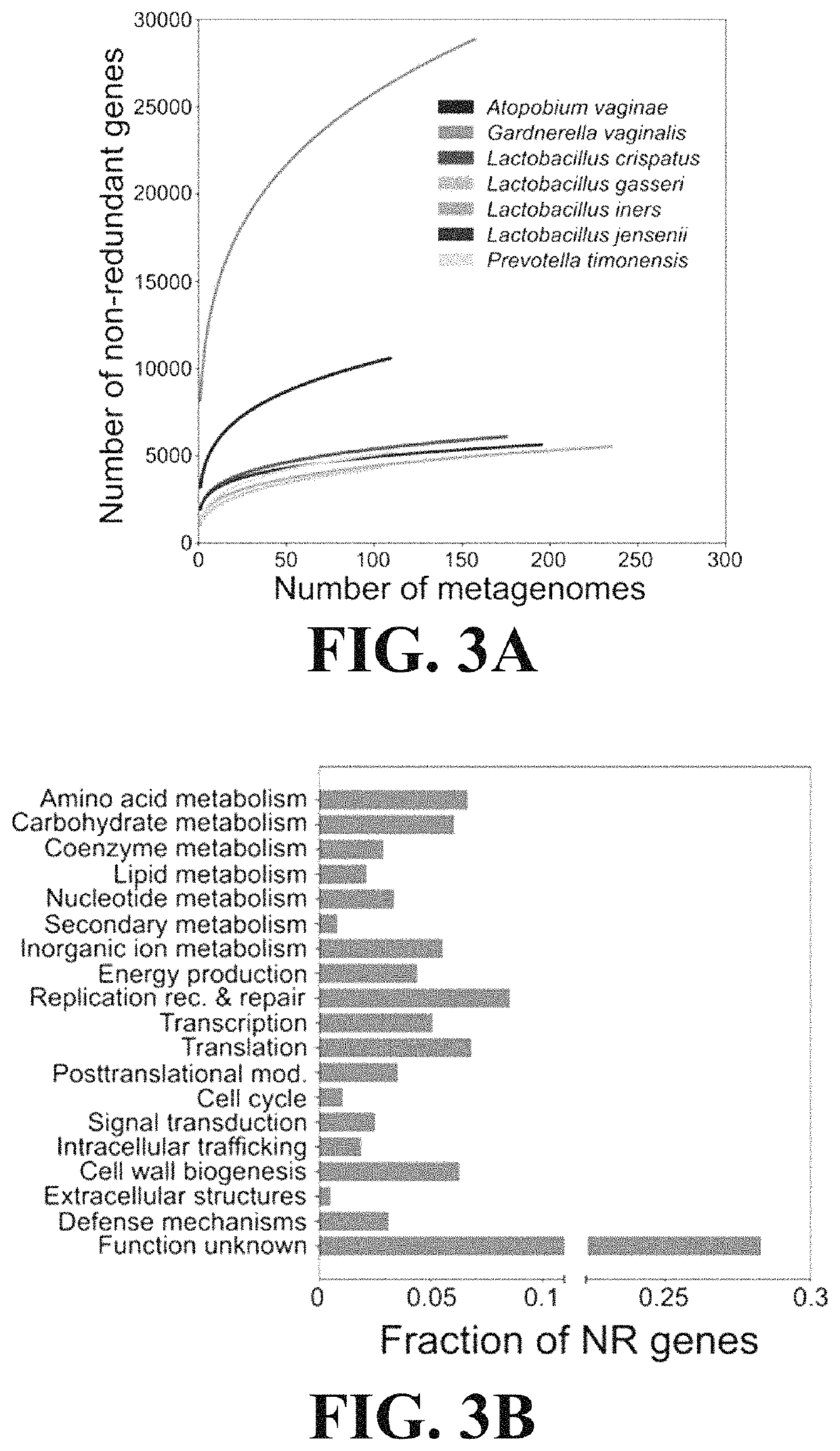
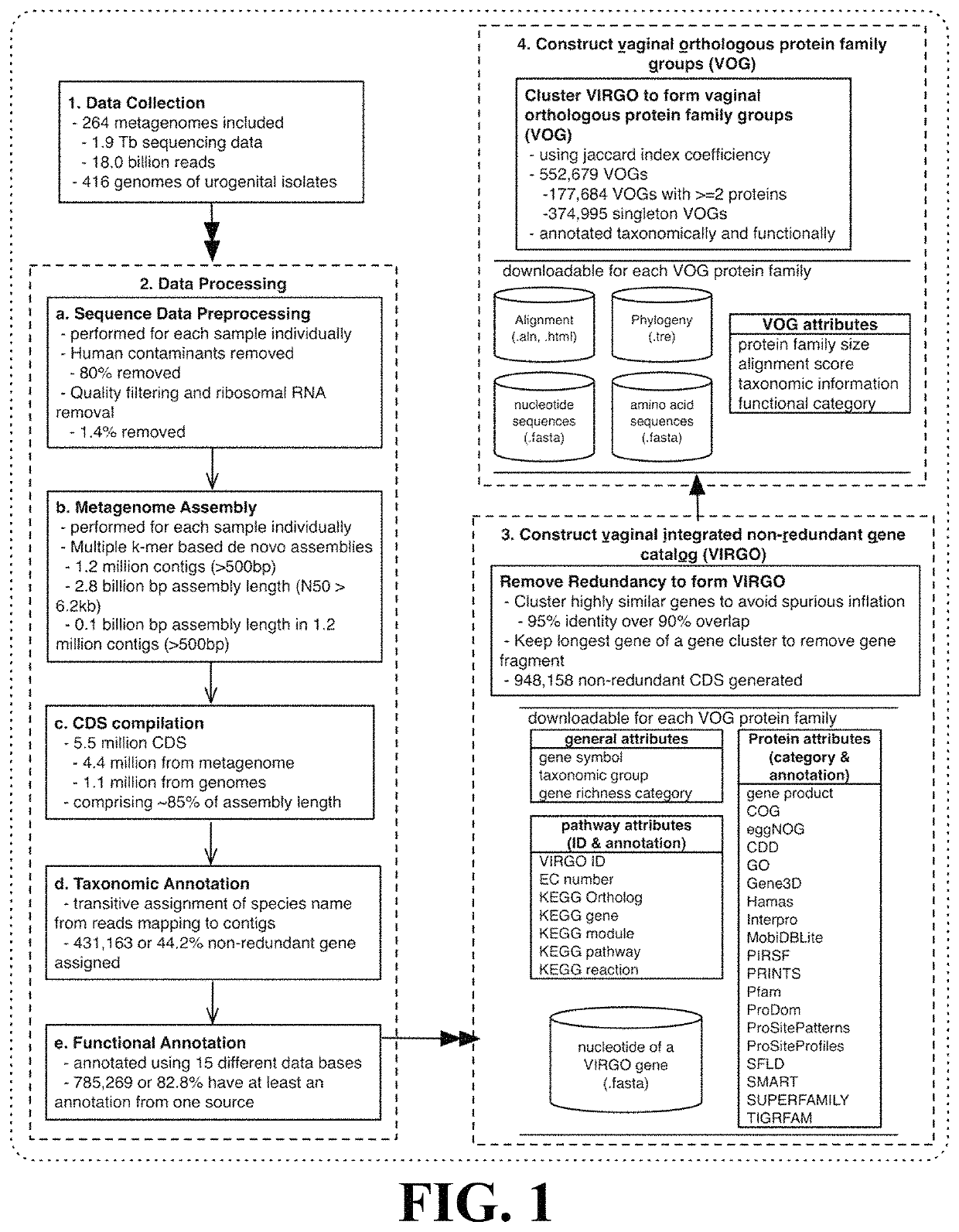
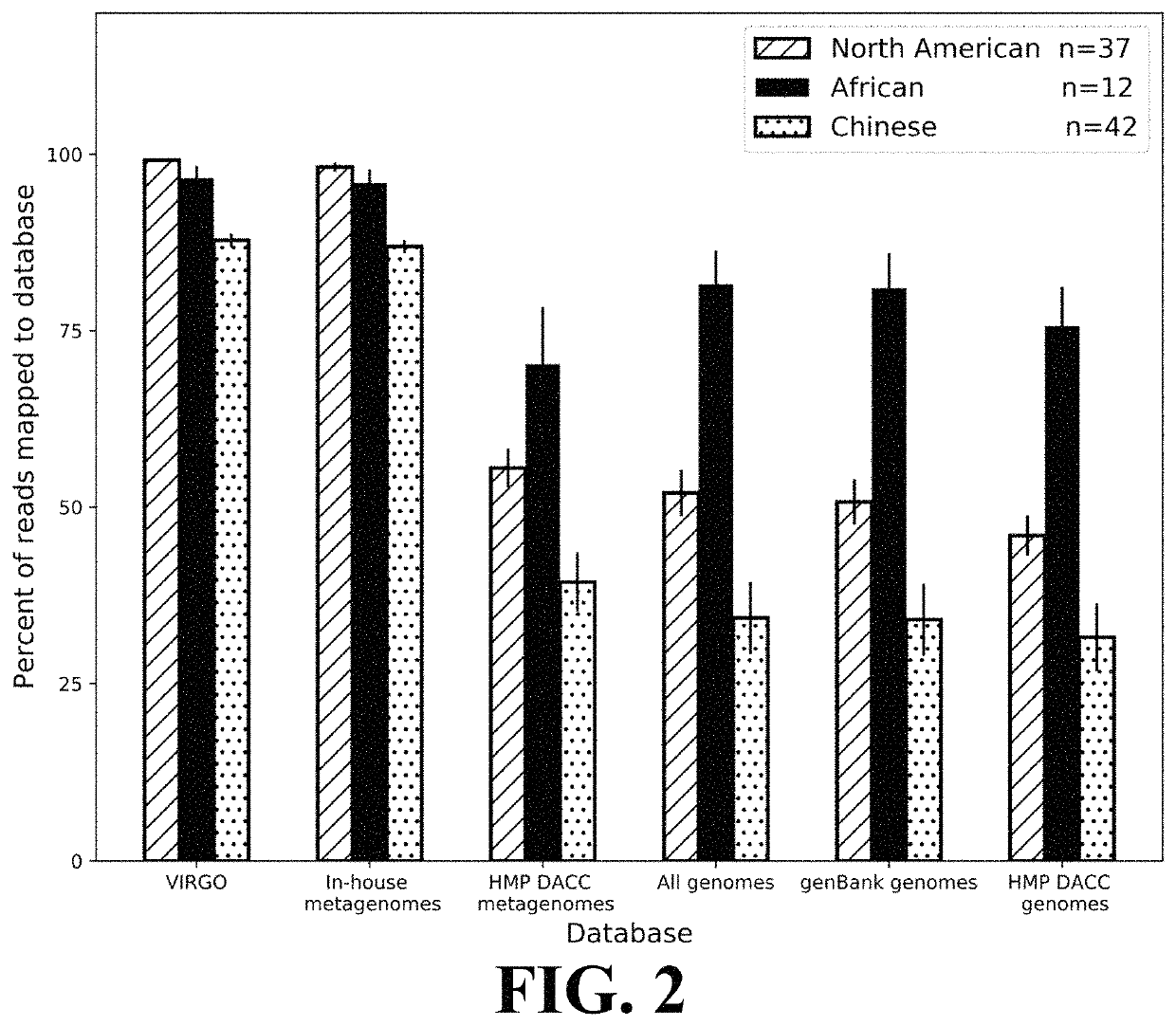
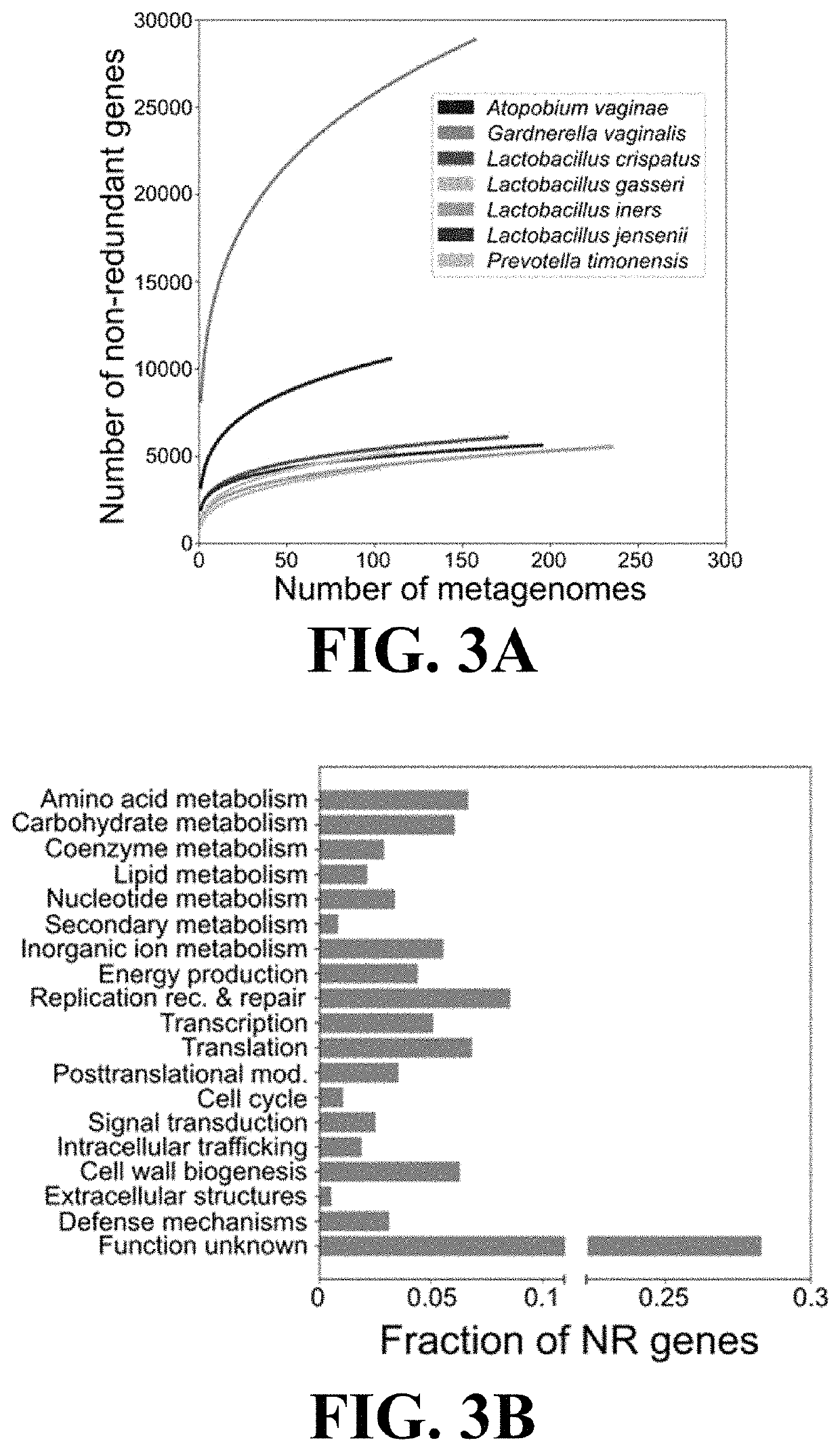
ActiveUS11037655B2Microbiome studyFast sequencingPeptide/protein ingredientsUnknown materialsVaginal microbiomeReference genes
Methods of formulating live biotherapeutics are disclosed in which a deficiency or excess of a specific bacterial strain in a person's microbiome is identified by comparing a gene-specific characterization of the person's microbiome against a comprehensive, non-redundant reference gene catalog, and the biotherapeutic is formulated by selecting bacteria to address the deficiency or excess. Embodiments include the formulation of live biotherapeutics for improving the health of a person's vaginal microbiome, i.e. using a vaginal reference gene catalog, and may be suitable for ameliorating, treating, or preventing a malignancy such as a cancer of the female genitourinary system.
Owner:UNIV OF MARYLAND
Epitope mimics
InactiveUS20190070255A1High binding affinitySsRNA viruses negative-sensePeptide librariesEpitopeBiotherapeutic agent
Owner:IOGENETICS
Torque sensitive diaphragm valve
InactiveUS20060006357A1Diaphragm valvesOperating means/releasing devices for valvesDiaphragm valveEngineering
Owner:MEYERS SCOTT CHRISTOPHER
Microbiome-based informed method to formulate live biotherapeutics
ActiveUS10967012B2Microbiome studyFast sequencingBacteriaPeptide/protein ingredientsVaginal microbiomeReference genes
Methods of formulating live biotherapeutics are disclosed in which a deficiency or excess of a specific bacterial strain in a person's microbiome is identified by comparing a gene-specific characterization of the person's microbiome against a comprehensive, non-redundant reference gene catalog, and the biotherapeutic is formulated by selecting bacteria to address the deficiency or excess. Embodiments include the formulation of live biotherapeutics for improving the health of a person's vaginal microbiome, i.e. using a vaginal reference gene catalog, and may be suitable for ameliorating, treating, or preventing a malignancy such as a cancer of the female genitourinary system.
Owner:UNIV OF MARYLAND
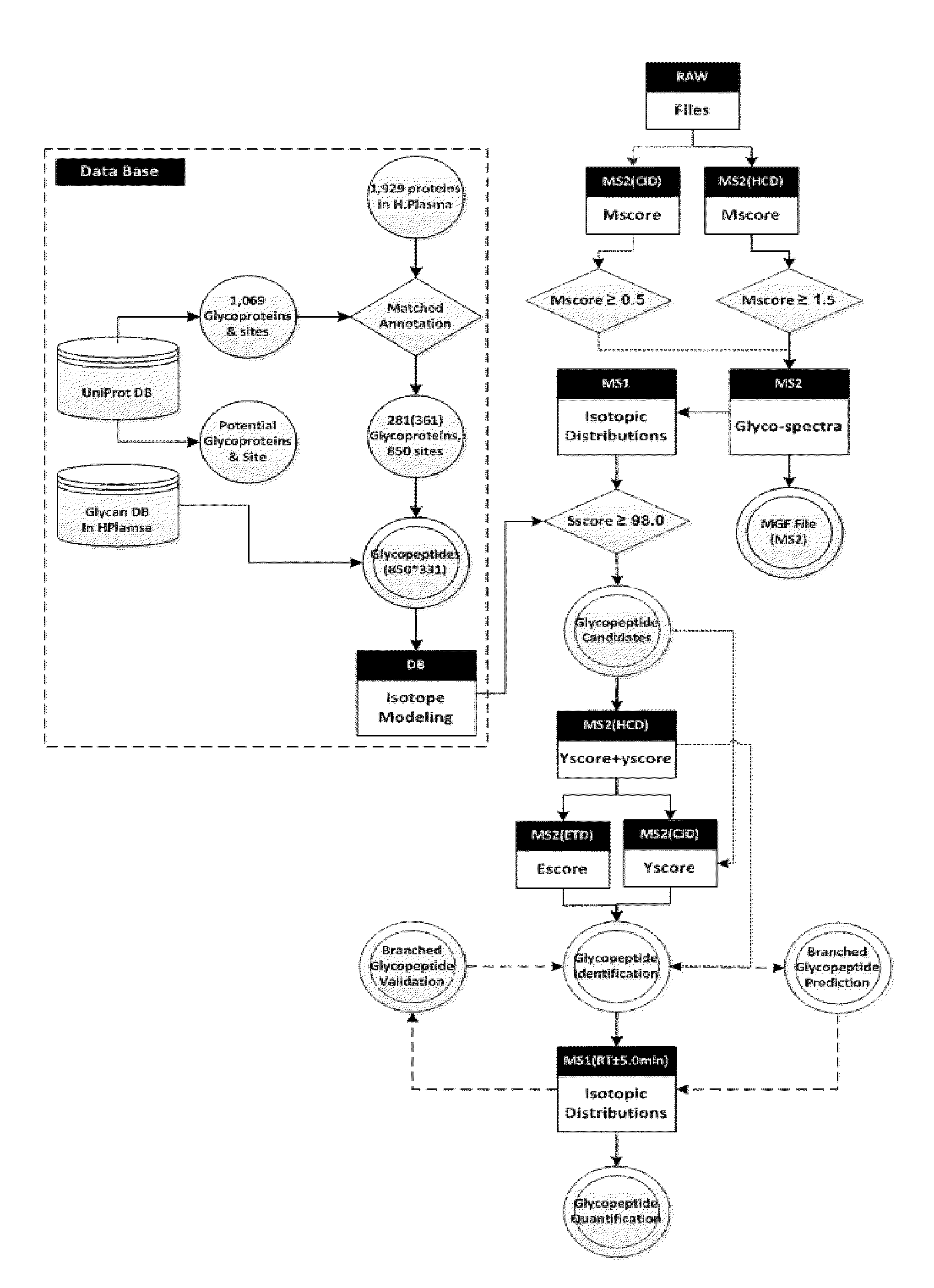
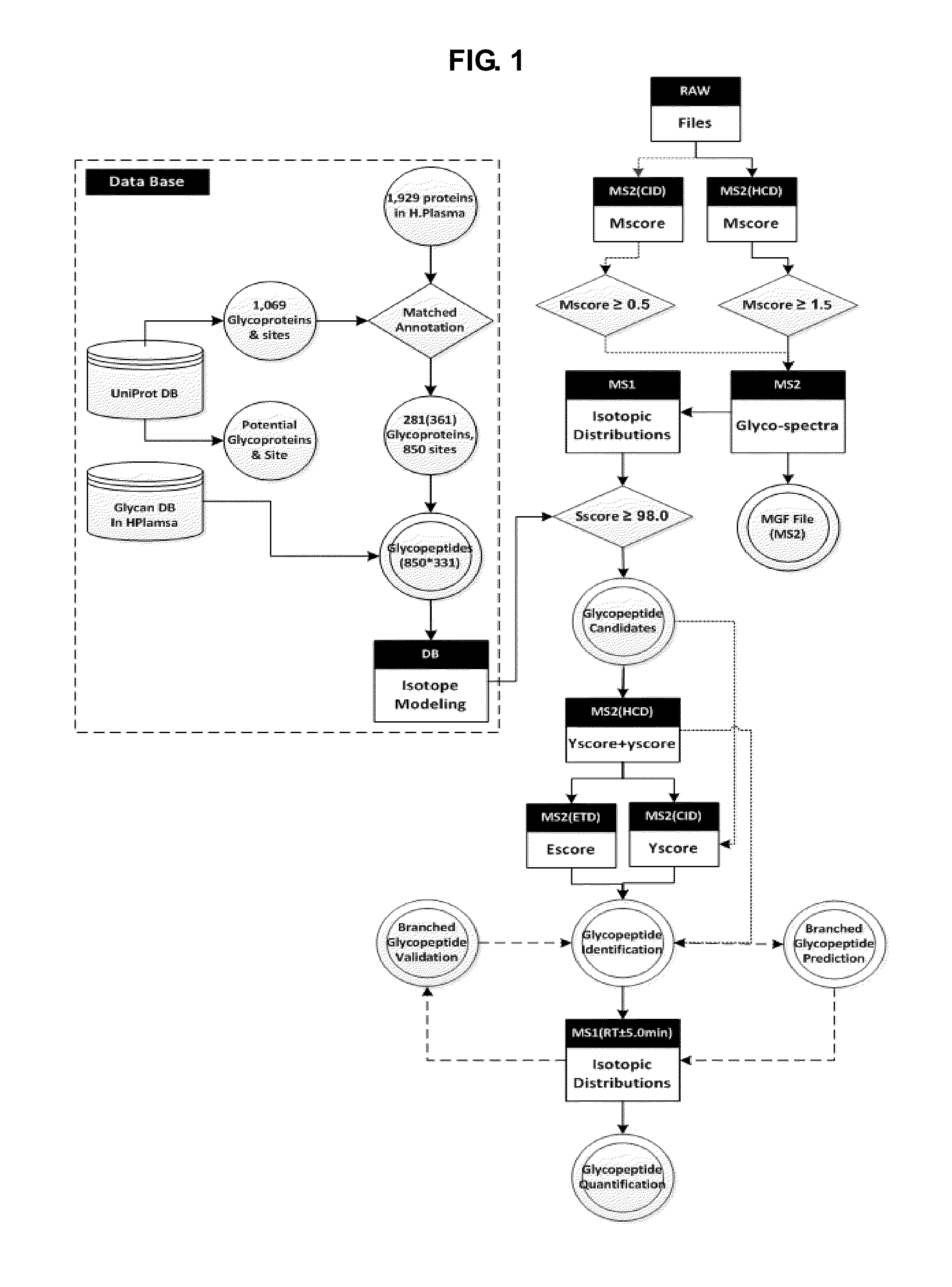
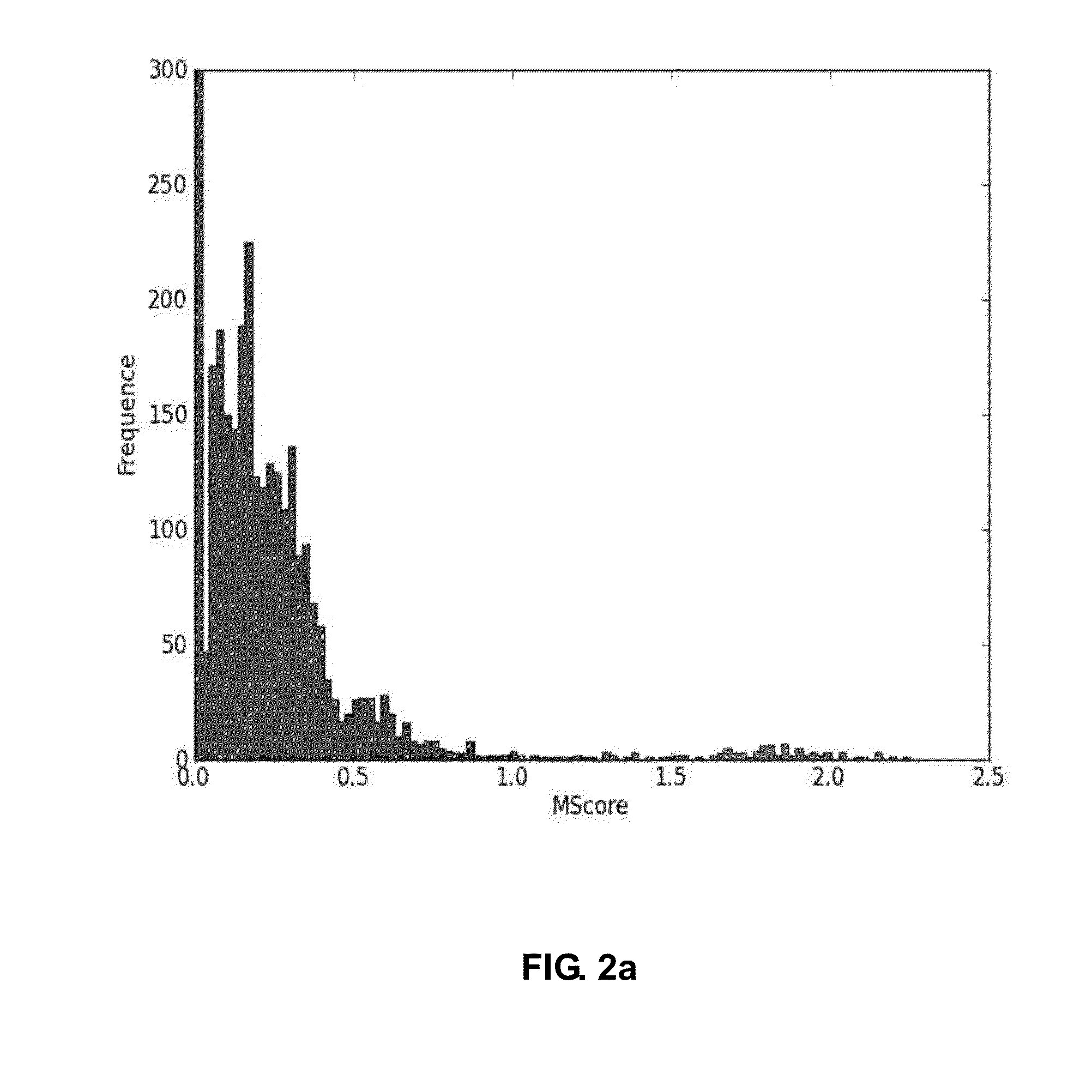
Bioinformatics Platform for High-Throughput Identification and Quantification of N--Glycopeptide
ActiveUS20150186595A1Efficient and accurateEasy to useComponent separationMicrobiological testing/measurementDiseaseGlycopeptide
The present invention relates to a more efficient and accurate method for the identification and quantification of comparatively low abundant glycopeptides, compared with general peptides, using mass spectrum obtained by using high resolution mass spectrometer. Therefore, the method of the present invention can be effectively used for the techniques for identification of biotherapeutics and diagnosis of cancer or disease by screening glycopeptide, the disease marker (Biomarker), from various samples.
Owner:KOREA BASIC SCI INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com