Diglycidic ether derivative therapeutics and methods for their use
A compound, alkyl technology, applied in the field of cancer therapy and treatment, can solve problems hindering virtual docking drug development methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
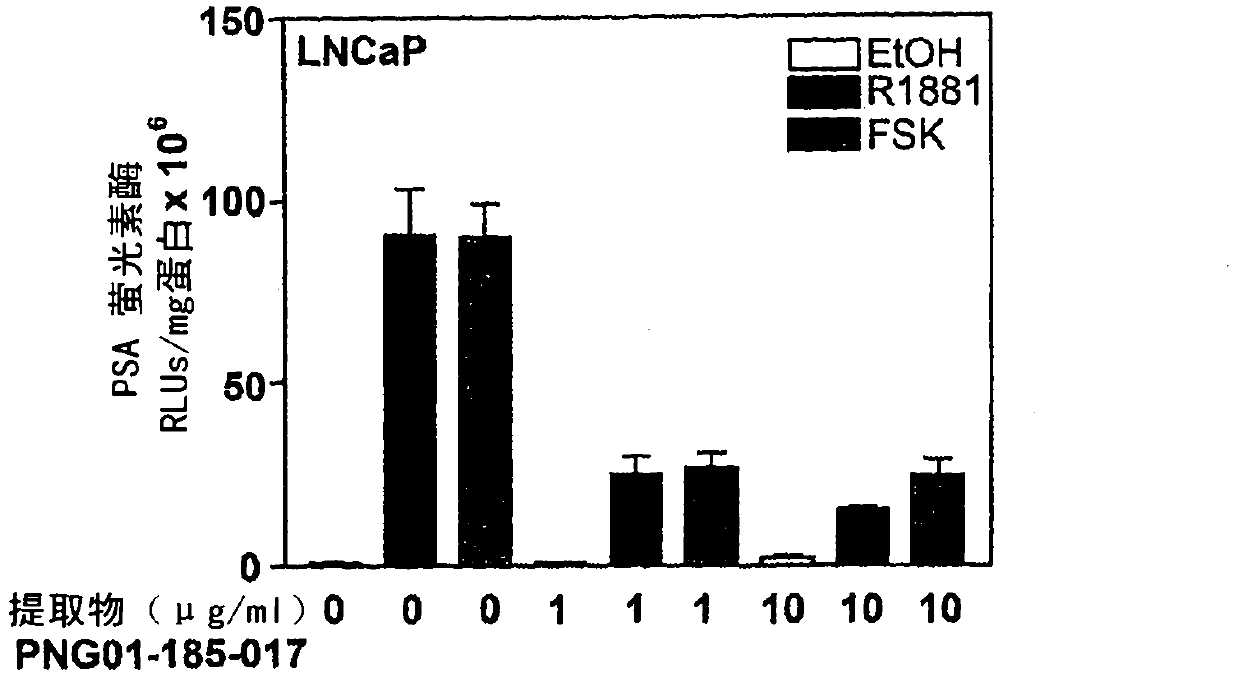
[0204] A number of screening applications are used to identify active compounds that inhibit AR NTD activity. The initial screen was a cell-based assay involving LNCaP cells maintained in culture. The assay consists of AR activation using both androgen (ligand-dependent) and forskolin (ligand-independent) and measuring the level of PSA secreted by LNCaP cells in the presence and absence of crude extract . PSA is an androgen-regulated gene containing several well-characterized AREs. Androgen-independent increase in PSA gene expression occurs through an AR-dependent mechanism in prostate cancer cells. PNG01-185 extract was observed to block PSA secretion induced by both androgen and forskolin.
[0205] To ensure that the inhibitory effect of PNG 01-185 extract on endogenous PSA protein was at the transcriptional level, reporter gene constructs were also investigated. Activation of endogenous AR was measured in LNCaP human prostate cancer cells by measuring an androgen-respon...
Embodiment 2
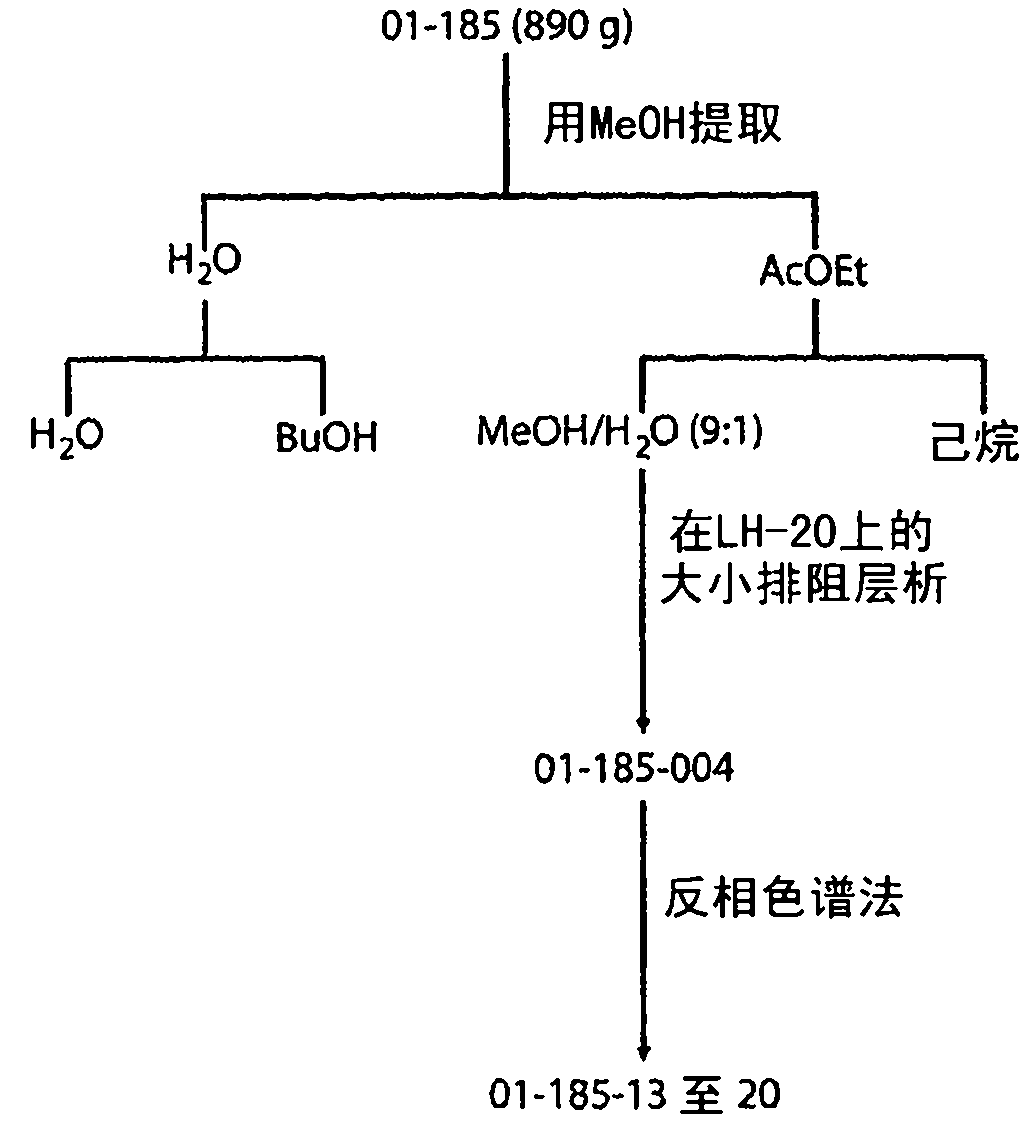
[0217] The purified active compound from the extract described in Example 1 was isolated. Geodia lindgreni (Lendenfeld, 1903) samples were collected by hand using SCUBA at a depth of 5 M below the rocks on a sheltered reef off Loloata Island, Papua New Guinea. The frozen sponge (890 g) was then exhaustively extracted with MeOH and the crude extract was observed to be active in the assay described in Example 1 above. Bioassay-guided fractionation provided purified samples PNG01-185-017-2, -5, -6, -7 and -8 ( figure 2 ). The structure has been elucidated by analysis of NMR and MS data, which are shown in Example 1 above.
Embodiment 3
[0220] Compounds were identified by applying secondary screening. Purified compounds were tested for their ability to inhibit: transactivation of the androgen receptor N-terminal domain (AR NTD); other steroid receptors (specificity); endogenous expression of PSA mRNA; interaction; N / C interaction; and proliferation of prostate cancer cells in response to androgens.
[0221] Transactivation of the AR NTD
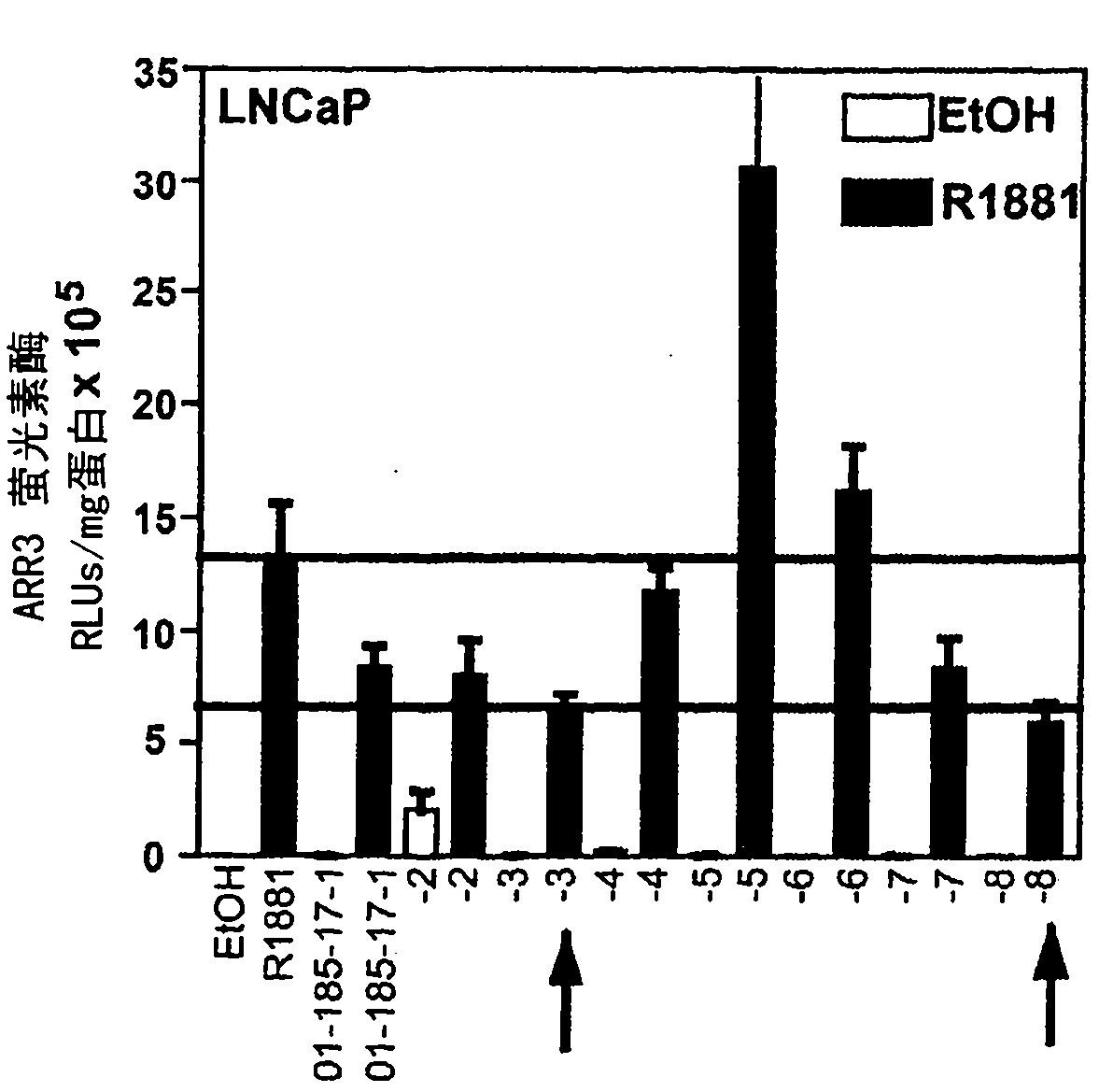
[0222] In the absence of serum and androgens, both forskolin (FSK) and IL-6, which stimulate PKA activity, increase PSA gene expression in prostate cancer cells through a mechanism involving AR NTD transactivation (Sadar, M.D., J.Biol.Chem. 274, 7777-7783 (1999); Ueda, T., Bruchovsky, N., Sadar, M.D., J.Biol.Chem. 277, 7076-7085 (2002); Ueda, T., Mawji, N.R., Bruchovsky, N., Sadar, M.D., J. Biol. Chem. 277, 38087-38094 (2002B); Quayle SN, Mawji NR, Wang J , Sadar M.D., Proc Natl Acad Sci U S A. 2007 Jan 23;104(4):1331-6.). The ability of 185-9-2 to inhibit transactivatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com