Probiotic bifidobacterium strain
A technology of bifidobacteria and strains, applied in bacteria, antibacterial drugs, antifungal agents, etc., can solve problems such as host injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
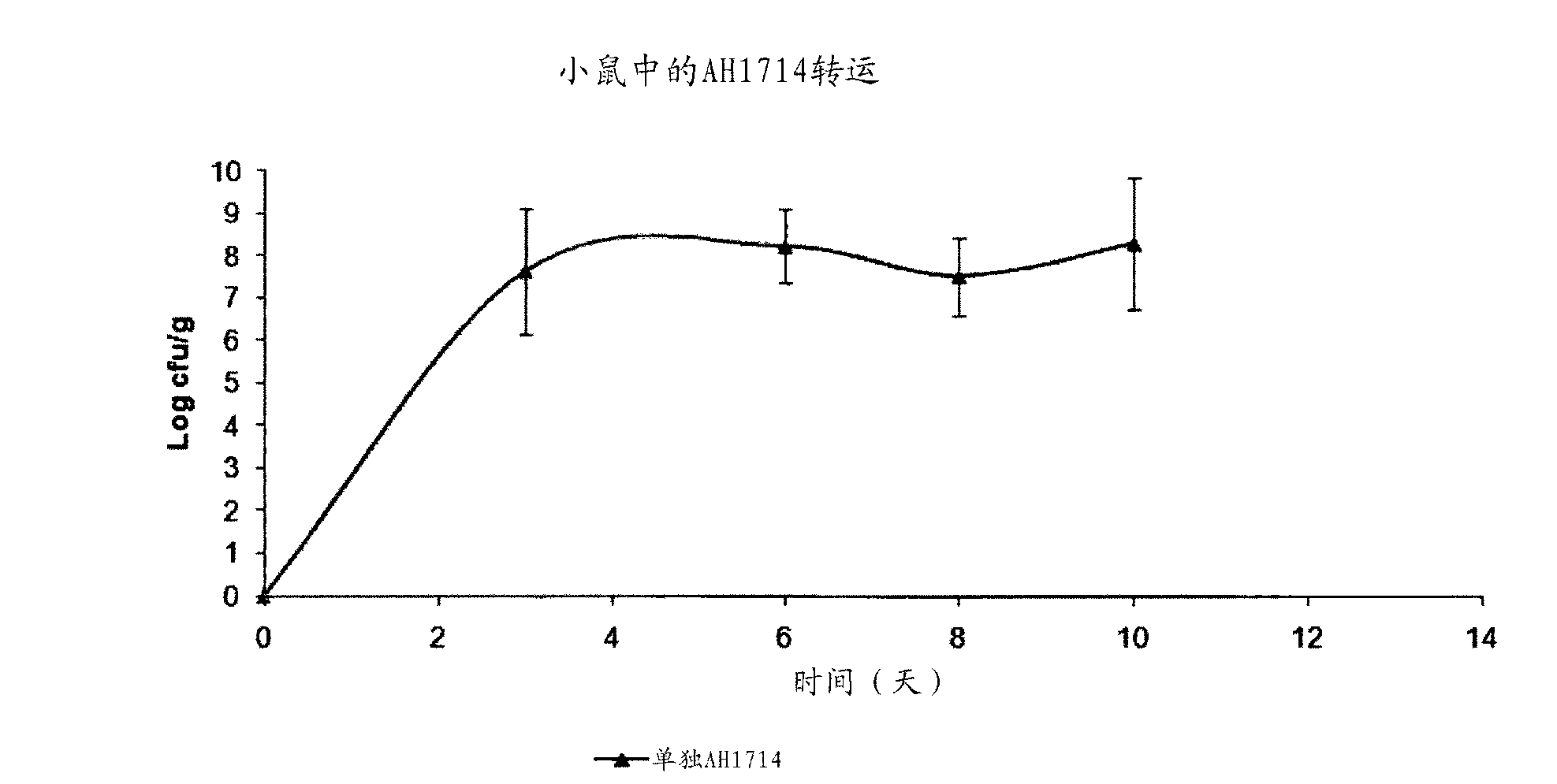
[0073] Example 1: Isolation of Bifidobacterium longum AH1714
[0074] Bifidobacterium longum strain AH1714 was isolated from biopsies of healthy human subjects.
[0075]The screen for probiotics used a large number of human gastrointestinal tissue sections obtained from colorectal cancer screening. The human gastrointestinal mucosal tissue was transferred to a test tube containing phosphate buffered saline (PBS) (supplemented with 0.05% cysteine hydrochloride). Triton X-100 (0.05%) was added to release adherent microorganisms in tissue samples. Tissue samples were then incubated for 10 minutes. Samples were vortexed and then plated on selective agar (De Man, Rogosa and Sharpe (MRS) agar + vancomycin and Wilkins-Chalgren agar + mupirocin, respectively) of adherent lactic acid isolated from gastrointestinal tissue bacilli and bifidobacteria. Colonies were isolated from the plate and streaked three times to ensure the purity of the clones. Microscopic examination, Gram s...
Embodiment 2
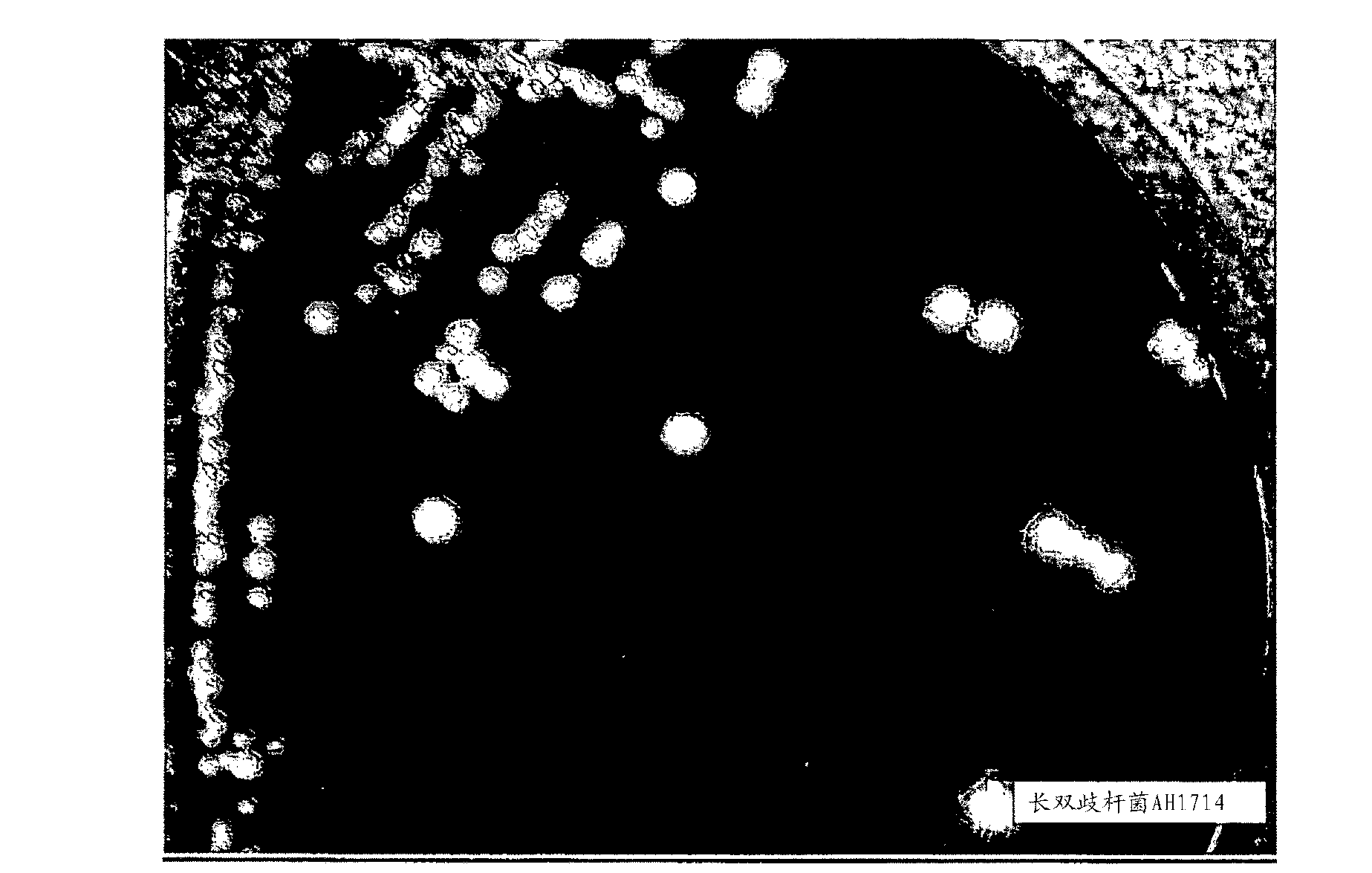
[0104] Example 2: Congo Red Agar Screening
[0105] Phenotypic screening of EPS-expressing bacterial strains was performed using Congo red agar selection. Briefly, 10 ml of modified Rogosa broth medium (+0.05% cysteine) was aseptically inoculated with a new colony of the bacterial strain and grown anaerobically at 37°C until cloudy (about 16 to about 24 hours). Broth cultures were aseptically streaked onto Congo red agar plates and incubated anaerobically at 37°C for 48 hours. EPS is believed to be produced as a by-product of growth and / or metabolism in certain strains, and EPS prevents the uptake of the Congo red dye, resulting in a creamy / white colony morphology. Strains that produce less EPS readily take up the Congo red dye, resulting in a pink / red colony morphology. Strains that do not produce EPS are stained red and appear almost transparent against the red agar background.
[0106] refer to figure 2 , the colony morphology of Bifidobacterium longum strain AH1714 ...
Embodiment 3
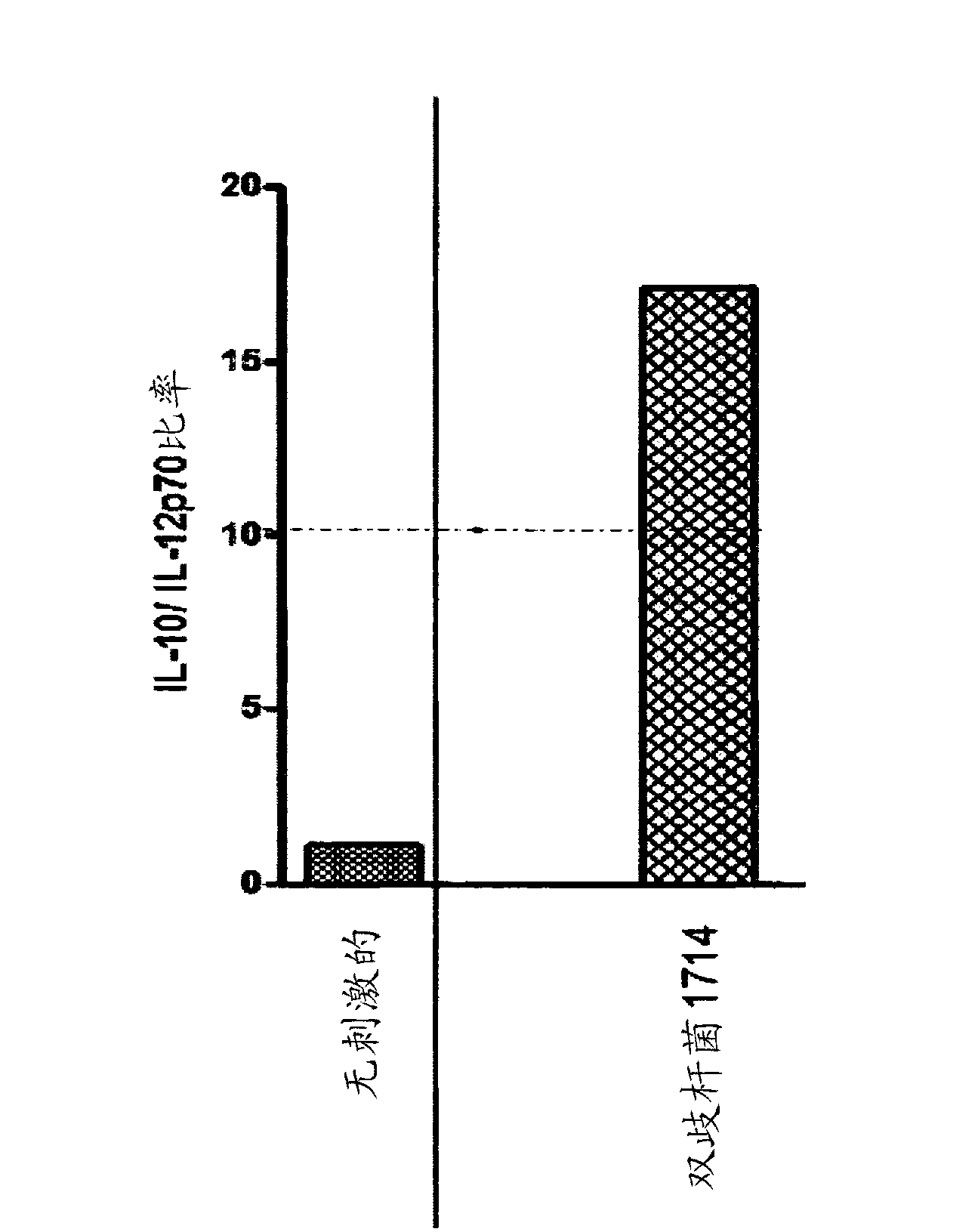
[0107] Example 3: Bifidobacterium strain 1714 induces a significantly elevated IL-10:IL-12 ratio .
[0108]Peripheral blood mononuclear cells (PBMC) were isolated from healthy human peripheral blood using BD Vacutainer CPT tubes (BD catalog 362761 ) according to the manufacturer's instructions. Wash PBMCs and resuspend them in Dulbecco's Modified Eagle Medium-Glutamax TM (Glutamax (glutamine substitute) + pyruvate + 4.5 g / l glucose (Gibco cat. no. 10569-010) 10% fetal bovine serum (Sigma cat. no. F4135), and 1% penicillin / streptomycin (Sigma cat. no. P0781). PBMC in (2×10 5 cells / well) in a flat-bottomed 96-well plate, and 20 μL of bacterial suspension (concentration of 1×10 7 CFU / mL). PBMC and bacteria at 37 °C / 5% CO 2 Co-culture in the incubator for 48 hours. After 2 days of culture, the plates were centrifuged at 300 xg, and the supernatant was removed and stored frozen at -80°C until analysis. Interleukin-10 (IL-10) and Interleukin-12p70 (IL-12p70) levels in cultur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com