Lactic Bacteria and Their Use in the Prevention of Diarrhea
a technology of lactic bacteria and aad, which is applied in the field of lactic bacteria strains in the prevention of diarrhea, can solve the problems of unavoidable development of effective preventive measures against aad, no bacterial strain has yet been shown to adhere in a permanent fashion, and the risk of aad not only for the patient undergoing antibiotic therapy, but also for the other patients, so as to prevent the occurrence of side effects, and prevent the aa side
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
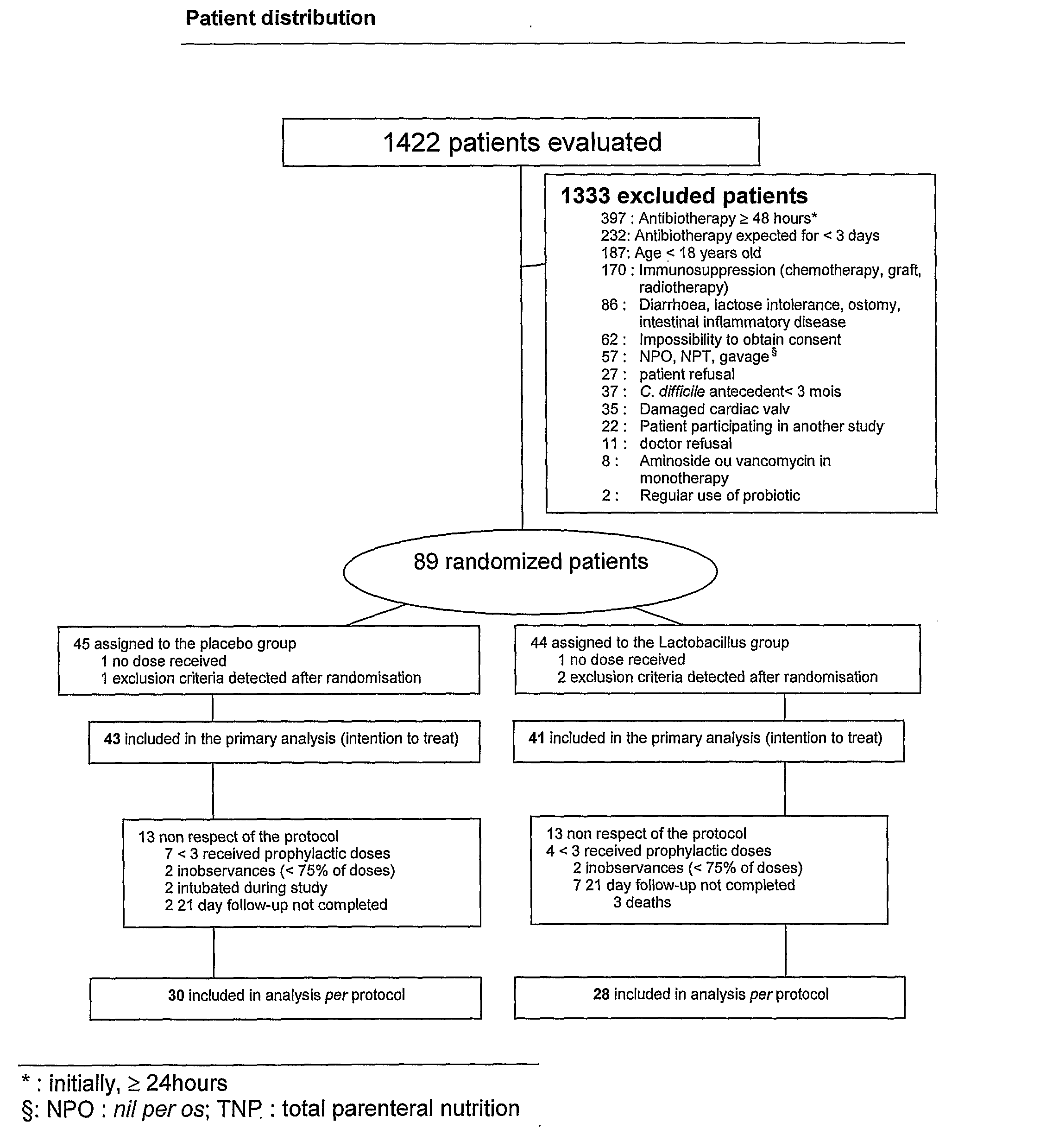
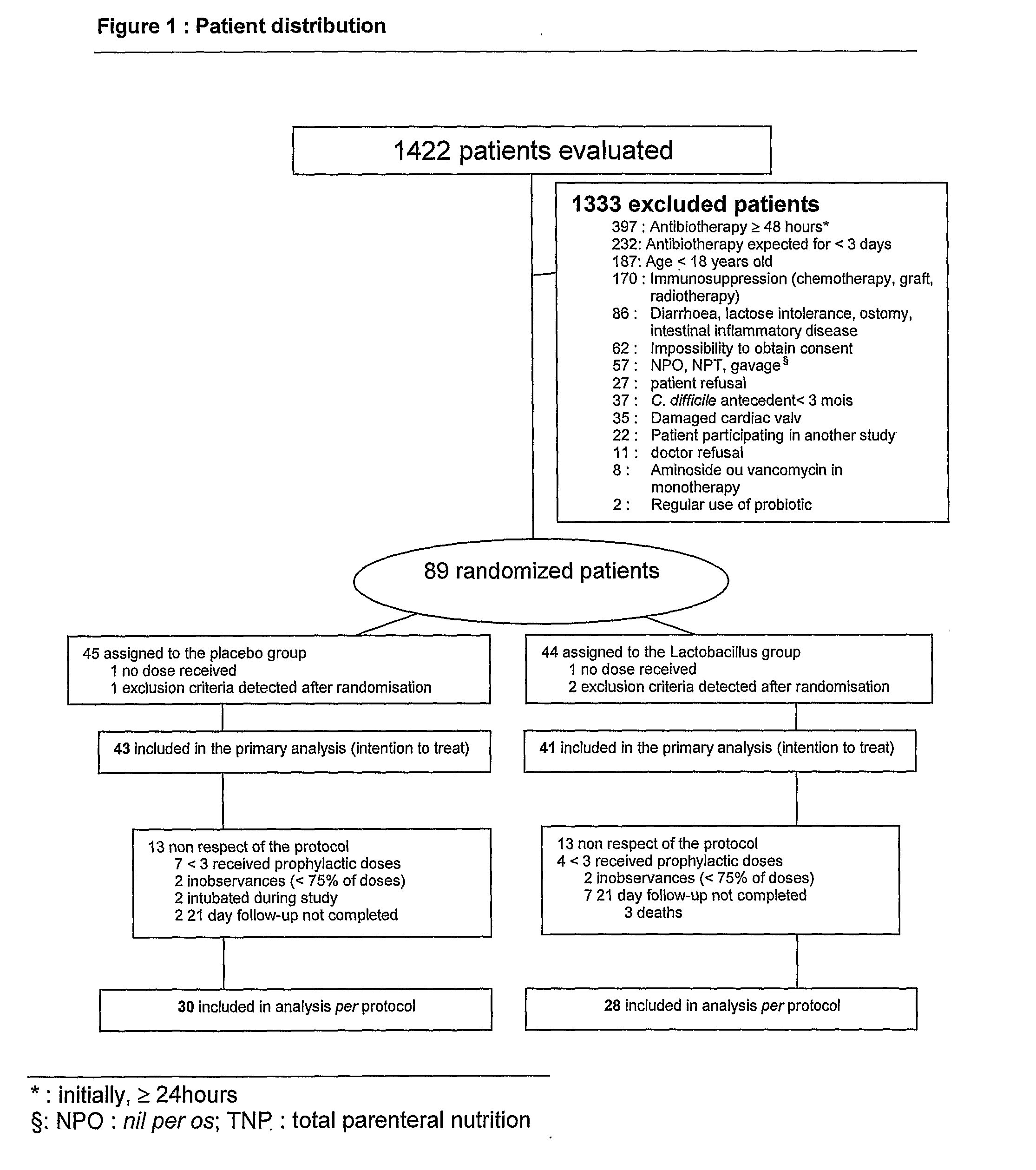
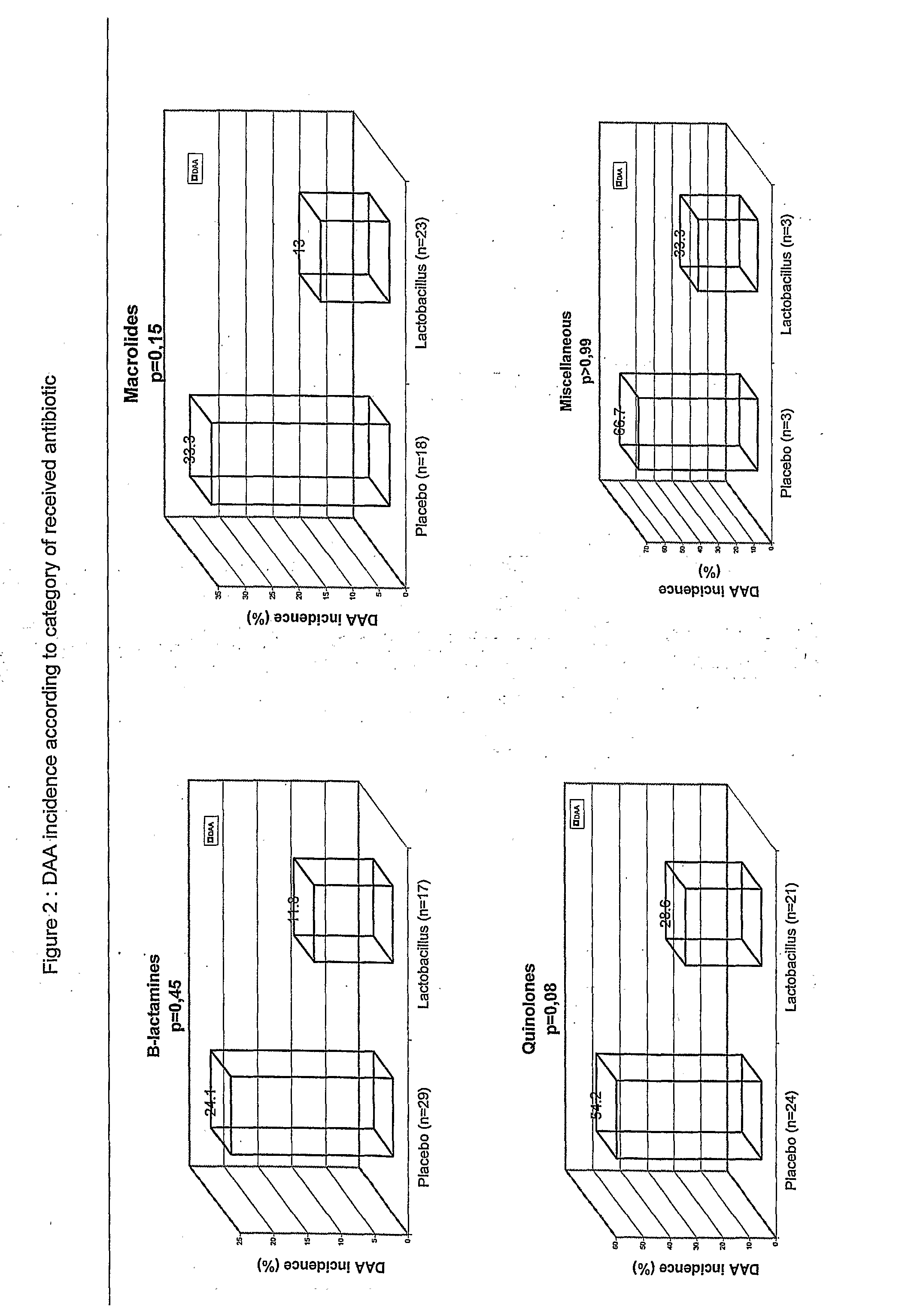
Study Comparing the Efficacy of a Preparation of lactobacillus (BIO-K+) to that of a Placebo in the Prevention of Antibiotic Associated Diarrhea
[0086] The study described in this example evaluates the utilization of a preparation of Lactobacillus in a lactic ferment in primary prophylaxis of AAD. Consequently, a double blind, randomized, placebo controlled clinical study was realized. Two groups are compared in this study: the experimental group receiving the preparation of Lactobacillus and the control group receiving the placebo preparation: whey devoid of any bacterial strain.
Population Studied
[0087] The hospitalized adult patients at the Maisonneuve-Rosemont hospital in Montreal, QC, Canada, receiving an antibiotic treatment orally or parenterally for an estimated period of a minimum of 3 days, other than an aminoside or a vancomycine in monotherapy, were eligible for the study. The exclusion criteria included: refusal to participate, impossibility to obtain consent, incapa...
example 2
Efficacy of Probiotics in Solving an Outbreak of Severe Clostridium difficile Colitis at the Pierre-Le Gardeur Hospital Centre
Introduction:
[0122]Clostridium difficile colitis is a frequent nosocomial infection in the Pierre-le Gardeur hospital centre (Montreal region, Quebec, Canada). Indeed, during the 2002-2003 fiscal year, the incidence was 9.5 cases / 1000 admissions. However, these infections, even the recurrent ones, did not present any severity and responded to a standard metronidazole or oral vancomycin treatment.
[0123] Between August and October 2003, there was nearly a 50% increase in the incidence of nosocomial cases with a severity and a mortality rarely encountered with this type of pathology. Furthermore, the response to the usual treatment was at times slow, and even without effect.
[0124] In November 2003, a series of measures to counter the situation were taken. The infected patients were isolated as a cohort with dedicated personnel. In addition, a more rigorous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com