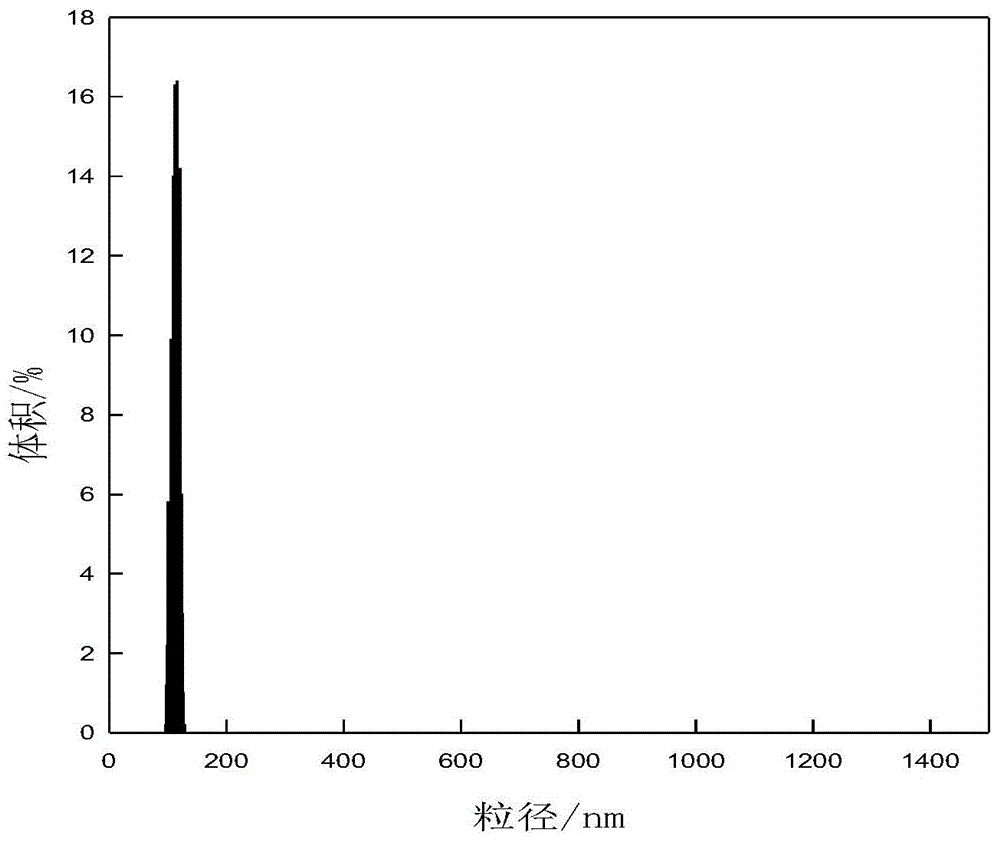
A kind of stable flurbiprofen axetil micro-nano emulsion and preparation method thereof
A technology of flurbiprofen axetil and micro-nano emulsion, which is applied in the direction of emulsion delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of easy demulsification, pain, and liver fat metabolism To avoid problems such as heavy burden, achieve good thermodynamic stability, reduce injection pain, and reduce vascular stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] prescription
[0040]
[0041] Process:
[0042](1) Preparation of the oil phase: Take 3.0g of egg yolk lecithin and 3g of polyethylene glycol-tocopherol palmitate, add them to 150g of soybean oil and 150g of medium-chain triglycerides, and stir at 30°C under the condition of nitrogen protection 20min to fully dissolve, then add 10g flurbiprofen axetil, dissolve and mix well, as the oil phase;
[0043] (2) Preparation of the water phase: under nitrogen protection and a temperature of 30°C, take 500 ml of water for injection, add 30 g of glycerin, stir to dissolve it, and use it as the water phase;
[0044] (3) Preparation of colostrum: under nitrogen protection and a temperature of 30°C, add the oil phase of step (1) into the water phase of step (2), high-speed shear dispersion, shear speed 10000rpm, time 15 minutes, to form Colostrum, and adjust the total amount to 1000ml with water for injection;
[0045] (4) High-pressure homogenization: Under the condition of ...
Embodiment 2
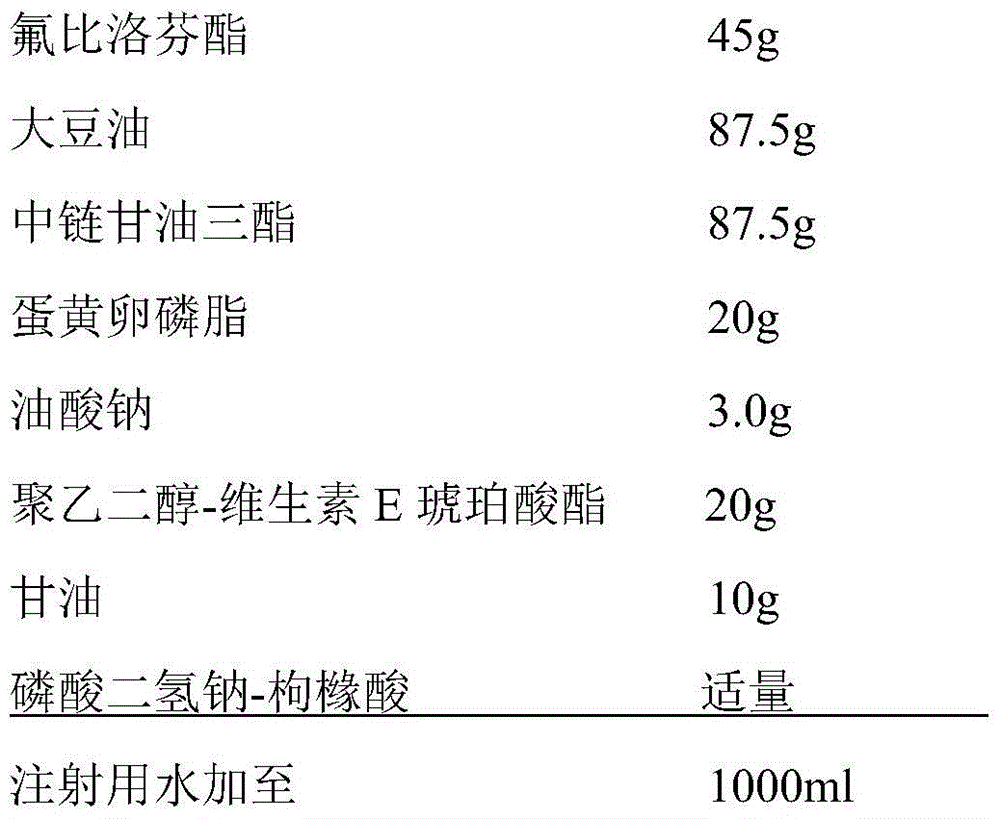
[0049] prescription
[0050]
[0051] Process:
[0052] (1) Preparation of the oil phase: Take 20g egg yolk lecithin, 3.0g sodium oleate, 20g polyethylene glycol-vitamin E succinate and add them to 87.5g soybean oil and 87.5g medium-chain triglycerides. Under the conditions, stir at 40°C for 20 minutes to fully dissolve, then add 45g of flurbiprofen axetil, dissolve and mix well, as the oil phase;
[0053] (2) Preparation of the water phase: under nitrogen protection and a temperature of 40°C, take 500 ml of water for injection, add 10 g of glycerin, stir to dissolve it, and use it as the water phase;
[0054] (3) Preparation of colostrum: under nitrogen protection and a temperature of 40°C, add the oil phase of step (1) into the water phase of step (2), high-speed shear dispersion, shear speed 8000rpm, time 35 minutes, to form Colostrum, and adjust the total amount to 1000ml with water for injection;
[0055] (4) High-pressure homogenization: Under nitrogen protection a...
Embodiment 3
[0058] prescription
[0059]
[0060] Process:
[0061] (1) Preparation of oil phase: Take 11g egg yolk lecithin, 6.0g sodium oleate, 40g polyethylene glycol-vitamin E palmitate, add 25g soybean oil and 25g medium chain triglyceride, under the condition of nitrogen protection , stirred at 20°C for 20 minutes to fully dissolve, then added 80 g of flurbiprofen axetil, dissolved and mixed evenly, as the oil phase;
[0062] (2) Preparation of the water phase: under nitrogen protection and a temperature of 20°C, take 500 ml of water for injection, add 50 g of glycerin, stir to dissolve it, and use it as the water phase;
[0063] (3) Preparation of colostrum: under nitrogen protection and a temperature of 20°C, add the oil phase of step (1) into the water phase of step (2), high-speed shear dispersion, shear speed 7000rpm, time 40 minutes, to form Colostrum, and adjust the total amount to 1000ml with water for injection;
[0064] (4) High-pressure homogenization: Under the con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com