Metoprolol succinate sustained-release tablet and preparation method thereof
A technology of metoprolol succinate and sustained-release tablets, which can be applied to pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the differences in drug release and release consistency defects, etc. problems, to achieve the effect of simplifying the production process, reducing the difficulty of process amplification, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
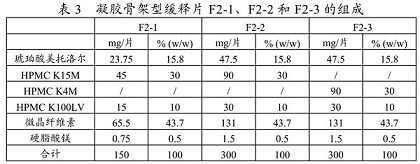
[0054] Metoprolol succinate hot-melt granules with different compositions and their preparation
[0055] Weigh each component of F3-1 to F3-6 prescription amount, mix each component evenly, and prepare the premixture into slow-release granules by hot-melt extrusion process under the condition of 100-110°C. See Table 5 for the compositions of F3-1 to F3-6 hot-melt particles.
[0056]
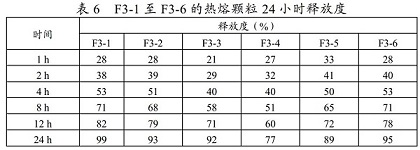
[0057] Weigh a certain amount of metoprolol succinate hot-melt granules from F3-1 to F3-6 (equivalent to 47.5 mg of metoprolol succinate), and measure the release rate of each prescription hot-melt granule within 24 hours . The results showed that the metoprolol succinate hot-melt granules prepared by using the selected molten materials could achieve the sustained-release effect in the 24-hour in vitro dissolution test. It can be used in the preparation of sustained-release solid preparations such as capsules or tablets.
[0058]
Embodiment 2
[0066] Preparation of Metoprolol Succinate Sustained-release Tablets by Direct Compression Tablets Using Hot-melt Granules
[0067] Use the metoprolol succinate hot-melt granule prepared in embodiment 1, take by weighing each component of F5-1 to F5-6 prescription amount respectively according to table 9, each prescription is mixed with each component except lubricant, and then Mix it again with an additional lubricant, and after mixing thoroughly, use a punch with a score to directly compress the tablet to prepare metoprolol succinate sustained-release tablets F5-1 to F5-6.
[0068]
[0069] Take metoprolol succinate sustained-release tablets F5-1 to F5-6, and measure the release rate of the sustained-release tablets before and after splitting along the notch direction. The measurement results are shown in Table 10. The results showed that metoprolol succinate sustained-release tablets F5-1 to F5-6 showed sustained-release effects in the 24-hour in vitro dissolution test, ...
Embodiment 3
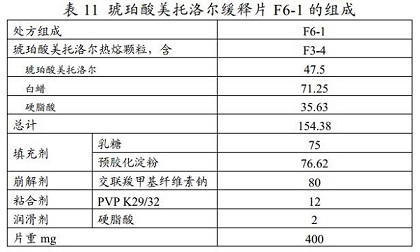
[0072] Preparation of metoprolol succinate extended-release tablets by wet granulation process using hot-melt granules
[0073] Weigh the hot-melt granules F3-4 prepared in Example 1, lactose, and pregelatinized starch according to the prescription in Table 11, and after mixing thoroughly, use PVP K29 / 32 aqueous solution to carry out wet granulation, and dry the obtained wet granulated granules. Sieve, then crush the granules on the sieve, and finally mix the granules under the sieve and the crushed granules on the sieve with the added lubricant stearic acid, and then use a punch with a score to perform tableting to prepare metoprolol succinate Sustained-release tablet F6-1.
[0074]
[0075] Take metoprolol succinate sustained-release tablet F6-1, and measure the release rate of the sustained-release tablet before and after splitting along the notch direction, and see Table 12 for the measurement results. The results showed that metoprolol succinate sustained-release tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com