Carbostyrile antibiotic injection preparations
A technology of quinolones and injection preparations, applied in the directions of antibacterial drugs, drug delivery, powder delivery, etc., can solve problems such as application limitations, and achieve the effects of good antibacterial effect, strong antibacterial effect, and broad antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Make the concentrated solution that is made into solution before use with the raw and auxiliary materials of the following weight ratio:
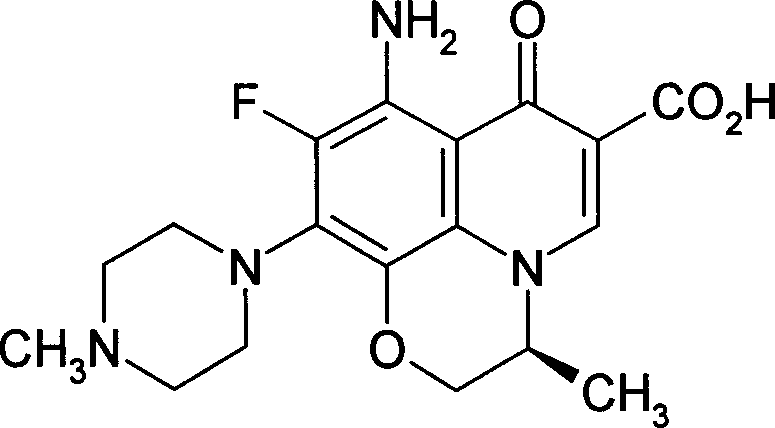
[0021] Quinolones
Embodiment 2
[0023] Make the concentrated solution that is made into solution before use with the raw and auxiliary materials of the following weight ratio:
[0024] Quinolones
0.1mol / L hydrochloric acid
51.0g (102% feed)
Appropriate amount
Add water for injection to 1000ml, pack into 125 sticks, 10ml each stick, each stick contains main
Medicine 400mg
[0025] The above example sample preparation process is as follows:
[0026] 1. Dosing: Take quinolone antibiotics according to the weight ratio, add water for injection (about 60°C) to the full amount, stir to dissolve, and adjust the pH to about 4.0 with 0.1mol / L hydrochloric acid. Add 0.2% activated carbon, keep stirring for 20 minutes. 0.45 μm microporous membrane to remove activated carbon, take the crude filtrate, measure the pH value of the solution and pass the content, then pass the crude filtrate through a microporous membrane with a pore size of 0.22 μm for fine filtration.
[0027] 2. Fillin...
Embodiment 3
[0031] The sterilizing solution for injection into the body is made with the raw and auxiliary materials in the following weight ratio:
[0032] Quinolones
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com