Lipid microballoon injection solution for progestational hormone drug and preparation method thereof
A technology of injection and microspheres, applied in pharmaceutical formulations, drug combinations, drug delivery, etc., can solve the problems of obvious pain, inaccurate dosage, redness and swelling, and achieve the effect of enriching choices and avoiding liver first-pass effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
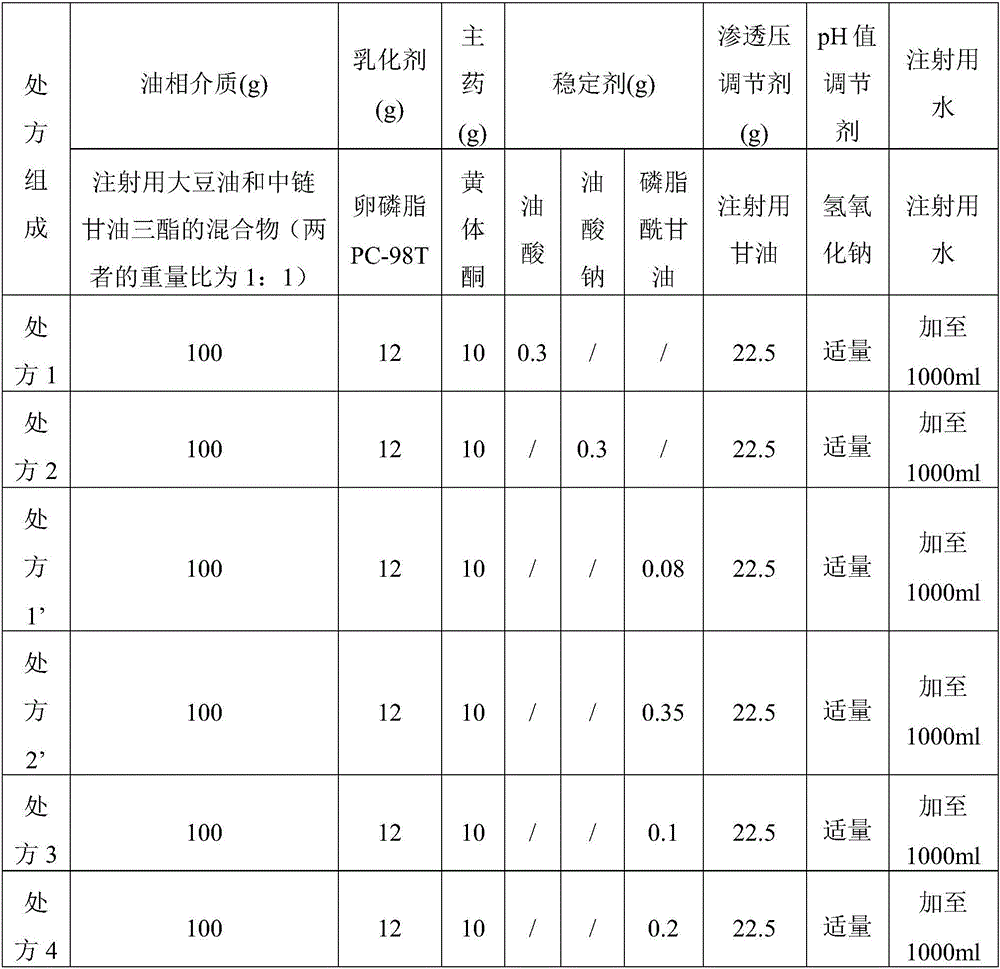
[0083] Example 1 Prescription screening of progesterone lipid microsphere injection
[0084] 1. Screening of stabilizers
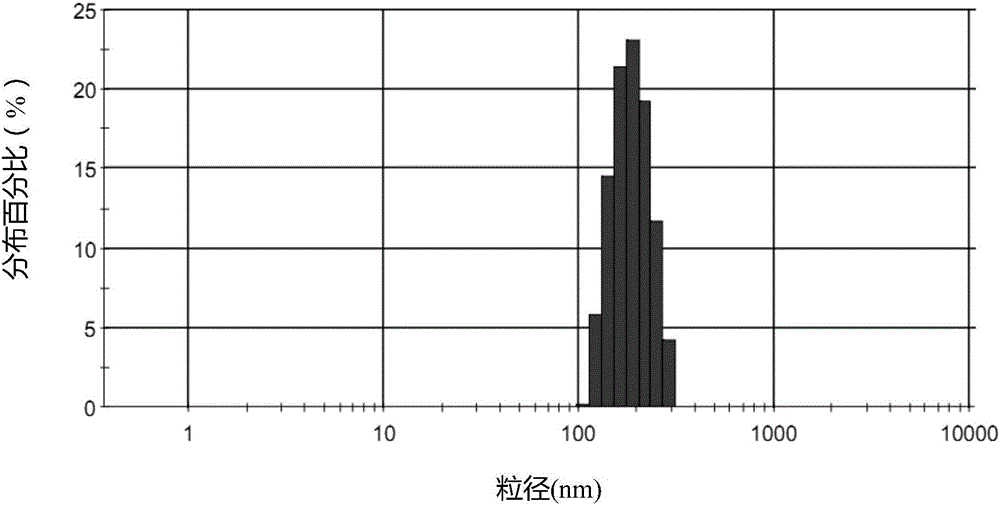
[0085] The stabilizer used in the lipid microsphere injection can increase the intermolecular force and the surface charge of the emulsion droplets, improve the physical stability of the emulsion through electrostatic repulsion, increase the strength of the lipid microsphere interface film, and increase the solubility of the drug. Therefore, it is necessary to investigate different stabilizers (phosphatidylglycerol, sodium oleate, oleic acid) and the dosage of stabilizers in the formulation. The average particle size, Di90, and PDI are closely related to the safety and stability of injectable emulsions. Under the same process conditions, formulations with smaller average particle size, Di90, and PDI usually have better physical stability. , Di90, and PDI are the inspection indexes, and the prescriptions containing different stabilizers are compared, and t...
Embodiment 2
[0103] Example 2 Preparation of Progesterone Lipid Microsphere Injection
[0104] prescription:
[0105]
[0106] Method:
[0107] (1) The mixture of soybean oil for injection and medium-chain triglyceride is heated to 70°C to 75°C, egg yolk lecithin PC-98T, egg yolk lecithin PL-100M, phosphatidyl glycerol, and progesterone are added, and the obtained The mixture is subjected to shearing treatment until the whole is dissolved and dispersed uniformly, so as to obtain the first mixture containing the oil phase of progesterone.
[0108] (2) The water for injection is heated to 70° C., glycerol for injection is added, and the resulting mixture is stirred well, so as to obtain a second mixture that forms an aqueous phase.
[0109] (3) Mixing the first mixture and the second mixture, shearing while mixing, rotating at 15000 rpm for 15 minutes, so as to obtain a third mixture for forming colostrum.
[0110] (4) The pH value of the third mixture is adjusted to 7.0 with sodium hy...
Embodiment 3
[0124] Example 3 Preparation of Progesterone Lipid Microsphere Injection
[0125] prescription:
[0126]
[0127]
[0128] Method:
[0129] (1) The mixture of soybean oil for injection and medium-chain triglyceride is heated to 70°C to 75°C, egg yolk lecithin PC-98T, egg yolk lecithin PL-100M, phosphatidyl glycerol, and progesterone are added, and the obtained The mixture is subjected to shearing treatment until the whole is dissolved and dispersed uniformly, so as to obtain the first mixture containing the oil phase of progesterone.
[0130] (2) The water for injection is heated to 70° C., glycerol for injection is added, and the resulting mixture is stirred well, so as to obtain a second mixture that forms an aqueous phase.
[0131] (3) Mixing the first mixture and the second mixture, shearing while mixing, and rotating at 10,000 rpm for 30 minutes, so as to obtain a third mixture for forming colostrum.
[0132] (4) The pH value of the third mixture is adjusted to 8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com