Ubenimex capsule medicament composition and preparation method thereof
A technology of Ubenimex and its composition, which is applied in the field of Ubenimex capsule pharmaceutical composition and its preparation, can solve the problems of high production cost, unfavorable large-scale production, complicated preparation process, etc., and achieve production cost reduction and safety Improvement, good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
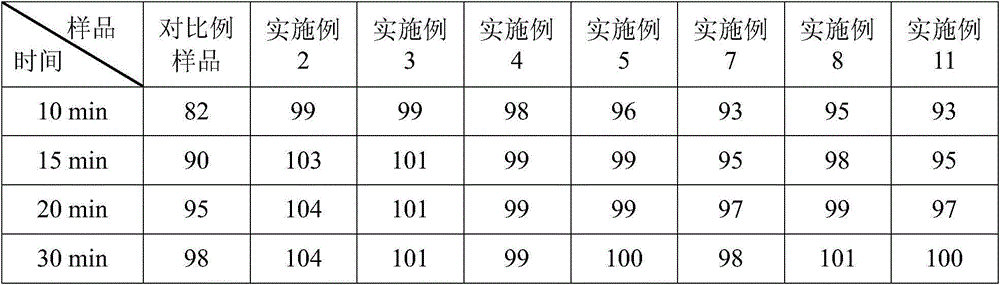
Examples
Embodiment 1
[0039] Example 1 Prescription of Ubenimex Capsules: (Based on 1000 capsules, unit: g)
[0040] Ubenimex
10.0g
Sucrose Fatty Acid Ester
0.8g
35.0g
125.0g
0.3g
[0041] Preparation Process:
[0042] (1) Mixing: First add the prescribed amount of microcrystalline cellulose, sucrose fatty acid ester and prescribed amount of Ubenimex into the hopper of the mixer in sequence, and perform the mixing operation on the mixer for 10 minutes; then add lactose into the hopper of the above mixer In the process, perform the mixing operation on the mixer for 30 minutes; finally, magnesium stearate is added in the hopper, and mixed for 15 minutes to obtain the total blend powder;
[0043] (2) Capsule filling: Add Ubenimex capsule blend powder into the lower hopper of the capsule filling machine to fill the capsules.
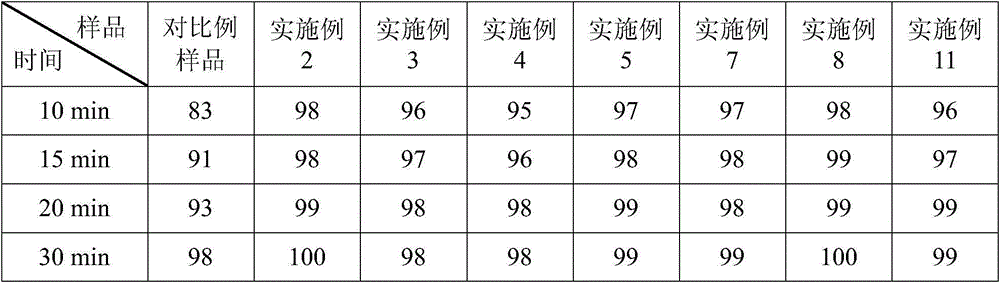
Embodiment 2
[0044] Example 2 Prescription of Ubenimex Capsules: (Based on 1000 capsules, unit: g)
[0045] Ubenimex
10.0g
Sucrose Fatty Acid Ester
1.7g
52.5g
105.0g
0.8g
[0046] Preparation Process:
[0047] (1) Mixing: first add the prescribed amount of microcrystalline cellulose, sucrose fatty acid ester and prescribed amount of Ubenimex into the hopper of the mixer in sequence, and perform the mixing operation on the mixer for 5 minutes; then add lactose into the hopper of the above mixer In the process, carry out the mixing operation on the mixer for 20 minutes; finally, magnesium stearate is added in the hopper, and mixed for 5 minutes to obtain the total blend powder;
[0048] (2) Capsule filling: Add Ubenimex capsule blend powder into the lower hopper of the capsule filling machine to fill the capsules.
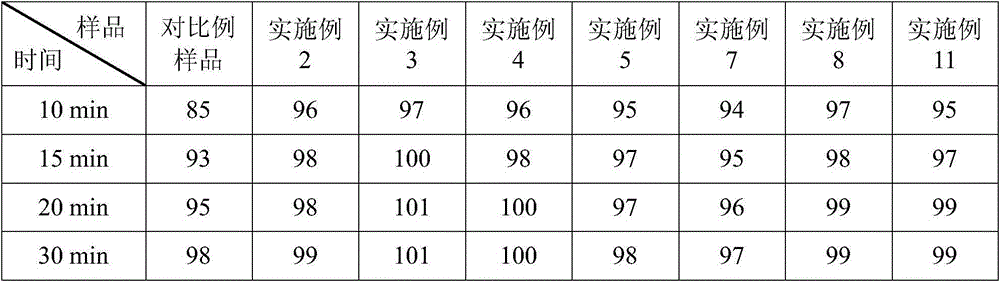
Embodiment 3
[0049] Example 3 Prescription of Ubenimex Capsules: (Based on 1000 capsules, unit: g)
[0050] Ubenimex
10.0g
Sucrose Fatty Acid Ester
1.2g
lactose
47.5g
100.0g
Magnesium stearate
0.5g
[0051] Preparation Process:
[0052] (1) Mixing: first add the prescribed amount of microcrystalline cellulose, sucrose fatty acid ester and prescribed amount of Ubenimex into the hopper of the mixer in sequence, and perform the mixing operation on the mixer for 25 minutes; then add lactose into the hopper of the above mixer In the process, perform the mixing operation on the mixer for 10 minutes; finally, magnesium stearate is added in the hopper, and mixed for 8 minutes to obtain the total blended powder;
[0053] (2) Capsule filling: Add Ubenimex capsule blend powder into the lower hopper of the capsule filling machine to fill the capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com