Pharmaceutical composition for treating respiratory system diseases and preparation method thereof
A technique for respiratory diseases and compositions, which is applied in the field of pharmaceutical compositions for treating respiratory diseases and its preparation, and can solve the problems of poor stability of terbutaline sulfate, poor stability of ipratropium bromide, and poor stability of terbutaline sulfate. Stability and other issues, to achieve the effect of improving the safety of medication, facilitating transportation and storage, and meeting the needs of clinical medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
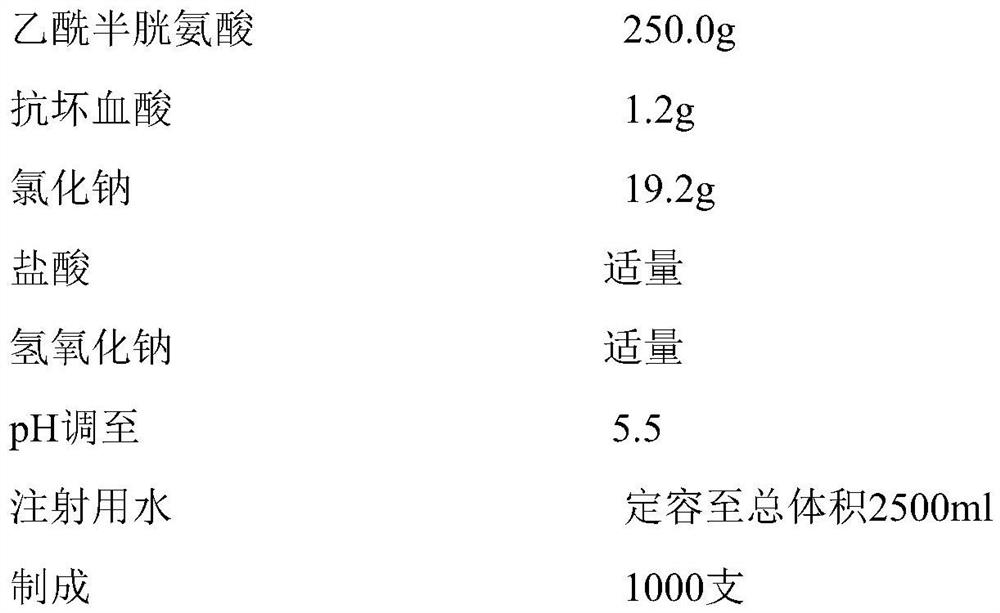
[0037]
[0038]
[0039] According to the prescription in Example 1a, add dispersion medium (water for injection), stabilizer (ascorbic acid), osmotic pressure regulator (sodium chloride), albuterol, acetylcysteine, acidic pH regulator in the liquid preparation tank successively, After stirring evenly, use an alkaline pH regulator to adjust the pH, the temperature of the water for injection is 20°C, and the product is aseptically treated by the sterilization and filtration production process, and the whole process is filled with nitrogen and repacked.
Embodiment 2a
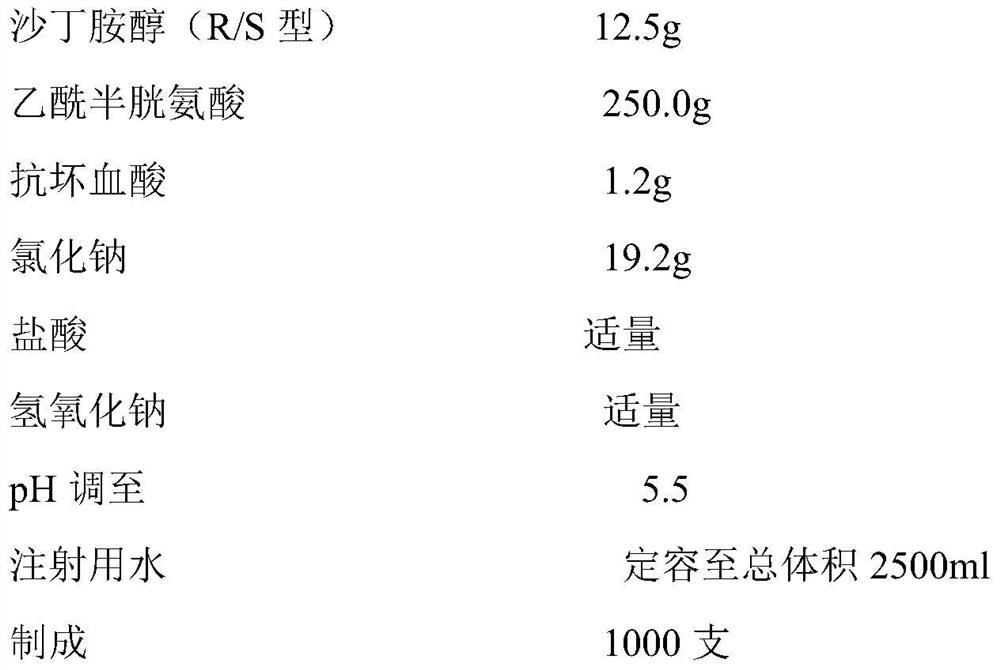
[0048]
[0049] According to the prescription in Example 2a, add dispersion medium (water for injection), stabilizer (ascorbic acid), osmotic pressure regulator (sodium chloride), albuterol, acetylcysteine, acidic pH regulator in the liquid preparation tank successively, After mixing evenly, use an alkaline pH regulator to adjust the pH, the temperature of the water for injection is 30°C, and the product is aseptically treated by the sterile filtration production process, and the whole process is filled with nitrogen for sub-packaging.
Embodiment 3a
[0051]
[0052]
[0053]According to the prescription in Example 3a, add dispersion medium (water for injection), stabilizer (ascorbic acid), osmotic pressure regulator (sodium chloride), albuterol, acetylcysteine, acidic pH regulator in the liquid preparation tank successively, After mixing evenly, use an alkaline pH regulator to adjust the pH, the temperature of the water for injection is 40°C, and the product is aseptically treated by the sterile filtration production process, and the whole process is filled with nitrogen for sub-packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com