Itopride hydrochloride medicine composition and preparation method thereof
A technology of itopride hydrochloride and its composition, which is applied in the field of itopride hydrochloride pharmaceutical composition and its preparation, can solve the problems that there is no development and application of itopride hydrochloride and simethicone composition, and achieve protection Gastrointestinal mucous membrane, elimination of gastrointestinal flatulence, and shortening of gastric emptying time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
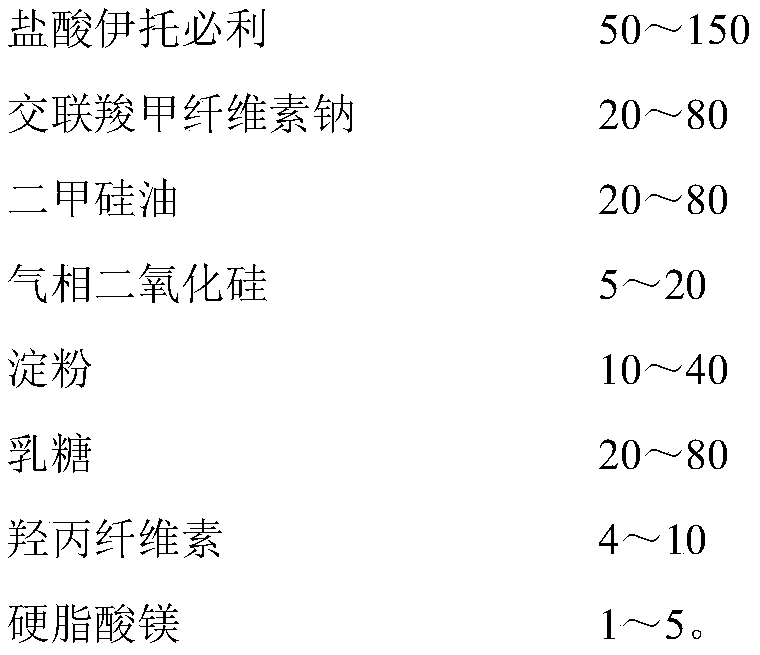
[0037] Itopride hydrochloride pharmaceutical composition formula is as shown in table 1 below:
[0038] Table 1
[0039] name mass (mg) Percentage, % Itopride Hydrochloride 50 38.5 Croscarmellose Sodium 20 15.4 Simethicone 20 15.4 fumed silica 5 3.8 starch 10 7.6 lactose 20 15.4 Hypromellose (EF or ELF) 4 3.1 Magnesium stearate 1 0.8
[0040] The preparation method of itopride hydrochloride pharmaceutical composition comprises the following steps:
[0041] A: Preparation of mixture of itopride hydrochloride and croscarmellose sodium
[0042] Itopride hydrochloride and croscarmellose sodium were grinded 4 times in a mass ratio of 2.5:1 with a planetary grinder (Model-PMRetsch Haan) at a speed of 200 rpm for 30 minutes each time, Collect and reserve;
[0043] B: Preparation of mixture of simethicone and fumed silica
[0044] Stir and mix simethicone and fumed silica at a mass ratio of 4:1 at 80°C to 150...
Embodiment 2
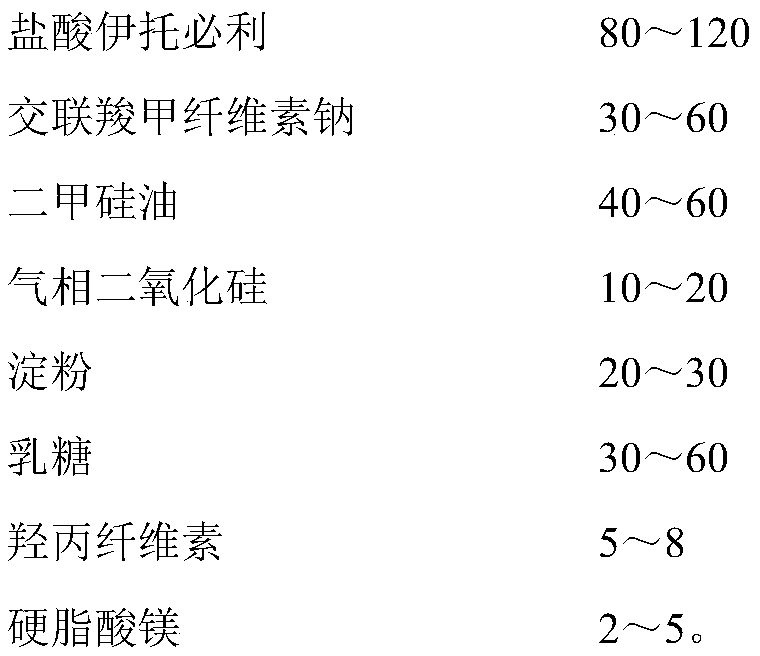
[0053] Itopride hydrochloride pharmaceutical composition formula is as shown in table 2 below:
[0054] Table 2
[0055] name mass (mg) Percentage, % Itopride Hydrochloride 100 38.5 Croscarmellose Sodium 40 15.4 Simethicone 40 15.4 fumed silica 10 3.8 starch 20 7.6 lactose 40 15.4 Hypromellose (EF or ELF) 8 3.1 Magnesium stearate 2 0.8
[0056] The preparation method of itopride hydrochloride pharmaceutical composition comprises the following steps:
[0057] A: Preparation of mixture of itopride hydrochloride and croscarmellose sodium
[0058] Itopride hydrochloride and croscarmellose sodium were grinded 4 times in a mass ratio of 2.5:1 with a planetary grinder (Model-PMRetsch Haan) at a speed of 200 rpm for 30 minutes each time, Collect and reserve;
[0059] B: Preparation of mixture of simethicone and fumed silica
[0060] Stir and mix simethicone and fumed silica at a mass ratio of 4:1 at 80°C to 1...
Embodiment 3
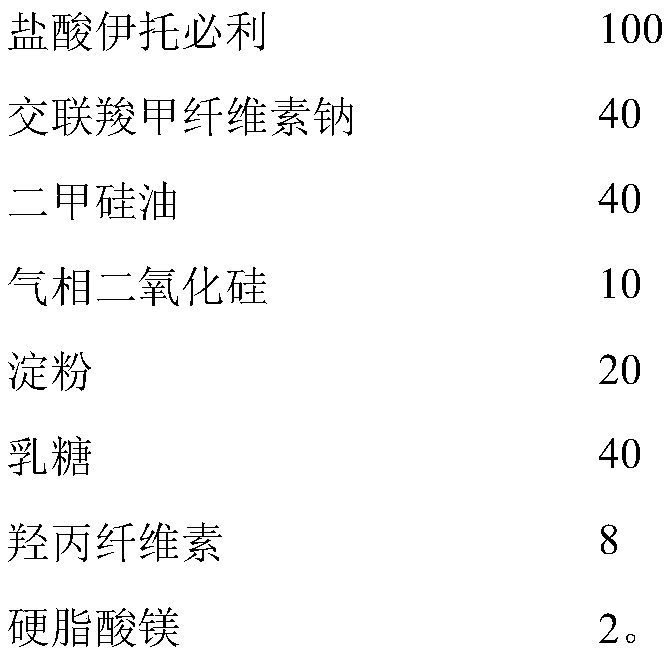
[0069] Itopride hydrochloride pharmaceutical composition formula is as shown in table 3 below:
[0070] table 3
[0071] name mass (mg) Percentage, % Itopride Hydrochloride 150 33.1 Croscarmellose Sodium 75 16.6 Simethicone 80 17.6 fumed silica 20 4.4 starch 40 8.8 lactose 75 16.6 Hypromellose (EF or ELF) 10 2.2 Magnesium stearate 3 0.7
[0072] The preparation method of itopride hydrochloride pharmaceutical composition comprises the following steps:
[0073] A: Preparation of mixture of itopride hydrochloride and croscarmellose sodium
[0074] Itopride hydrochloride and croscarmellose sodium were grinded 4 times in a mass ratio of 2:1 with a planetary grinder (Model-PMRetsch Haan) at a speed of 200 rpm for 30 minutes each time, Collect and reserve;
[0075] B: Preparation of mixture of simethicone and fumed silica
[0076] Stir and mix simethicone and fumed silica at a mass ratio of 4.4:1 at 80°C to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sedimentation volume | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com