Detection method of demethylated itopride nitrosamine
A technology of methyl-itopia nitramine and a detection method is applied in the detection field of desmethyl-itopia nitramine, can solve the problems of lack of data and the like, and achieves the effects of high sensitivity and good separation effect of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation of demethyl itopride nitramine is carried out according to the following reaction.
[0018]
[0019] Dissolve about 344mg of demethylitopride in 30mL of 1M HCl aqueous solution at room temperature, and slowly add 173mg of solid sodium nitrite, fully stir and dissolve, and react at room temperature for 3 hours. After the reaction was completed, it was extracted with dichloromethane, dried over sodium sulfate, filtered, and spin-dried to obtain about 296 mg of desmethyl-itopride nitramine as a solid.
[0020] 1 H NMR (400MHz, DMSO- d6 )δ 8.85 (t, J = 5.9 Hz, 1H ), 7.52 (dd, J = 8.4 Hz, J = 2.1 Hz 1H ), 7.48 (d, J = 2.1 Hz, 1H ), 7.24(m, 2H ), 7.01 (d, J= 8.4 Hz, 1H ), 6.90 (m, 2H ), 4.50 (m, 2H ), 4.40 (d, J = 5.9 Hz, 2H ), 4.29(m, 2H), 3.80 (d, J = 1.7, 6H), 3.05 (s, 2H).
[0021] 13 C NMR (100MHz, DMSO- d6 ) Δ165.6, 156.84, 156.78, 151.2, 148.2, 132.48,132.47, 128.7, 126.6, 114.5, 114.3, 110.9, 110.6, 63.5, 55.6,55.5, 52.3, 42.0, 40.0, 39.8, 39....
Embodiment 2
[0024] System Suitability Experiment
[0025] Solution preparation:
[0026] Diluent: Acetonitrile
[0027] Take a 15ml centrifuge tube, add 5.0ml of water and 5.0ml of acetonitrile, mix well, then add about 1.0g of anhydrous sodium sulfate, shake up and down 30 times immediately, vortex for 1min, let stand for 10min, take the upper layer to get a blank solution;
[0028] Take about 25mg of demethyl itopride nitramine reference substance, accurately weigh it, put it in a 25ml measuring bottle, add acetonitrile to dissolve and dilute to the mark, shake well; then accurately measure 200µl of the above solution, put it in a 25ml measuring bottle , add acetonitrile to dilute to the mark, and shake well; then accurately measure 200 µl of the above solution, put it in a 25ml measuring bottle, add acetonitrile to dilute to the mark, shake well to obtain a stock solution of demethylitopria nitramine. (64ng / ml).
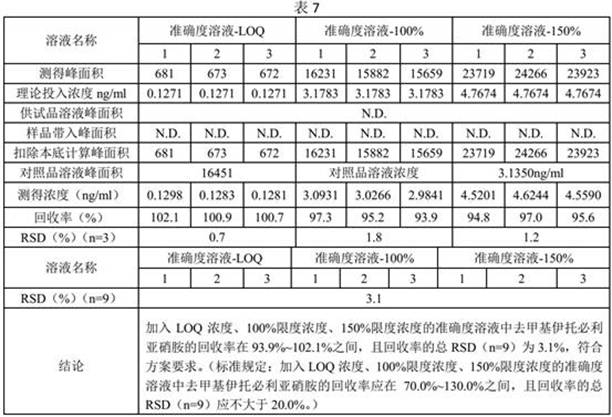
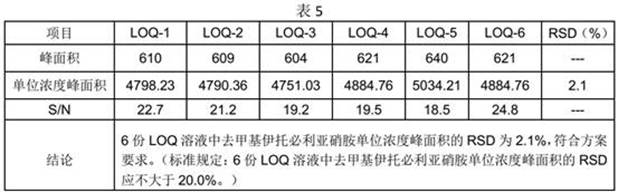
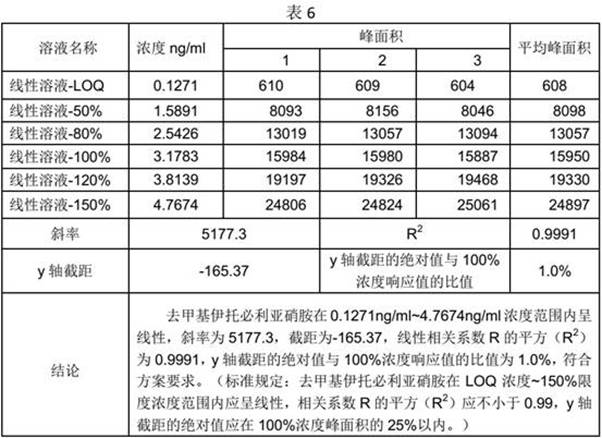
[0029] Precisely measure 2.5ml of the stock solution of demethyl itop...
Embodiment 3
[0040] specificity experiment
[0041] Solution preparation:
[0042] Diluent: acetonitrile;
[0043] Blank solution: Take a 15ml centrifuge tube, add 5.0ml of water and 5.0ml of acetonitrile, mix well, then add about 1.0g of anhydrous sodium sulfate, shake up and down 30 times immediately, vortex for 1min, let stand for 10min, and take the upper layer.
[0044] Demethyl itopride nitramine stock solution: take about 25 mg of demethyl itopride nitramine reference substance, weigh it accurately, put it in a 25ml measuring bottle, add acetonitrile to dissolve and dilute to the mark, shake well; Then precisely measure 200µl of the above solution, put it in a 25ml measuring bottle, add acetonitrile to dilute to the mark, and shake well; then precisely measure 200µl of the above solution, put it in a 25ml measuring bottle, add acetonitrile to dilute to the mark, and shake well. (Concentration: 64ng / ml)
[0045] Reference substance stock solution: Accurately measure 2.5ml of demet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com