A kind of itopride hydrochloride composition
A technology of itopride hydrochloride and tablet cores, applied in the field of itopride hydrochloride controlled-release tablet composition, can solve the problems that the release degree cannot reach 70%, cannot meet the clinical requirements of controlled-release preparations, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
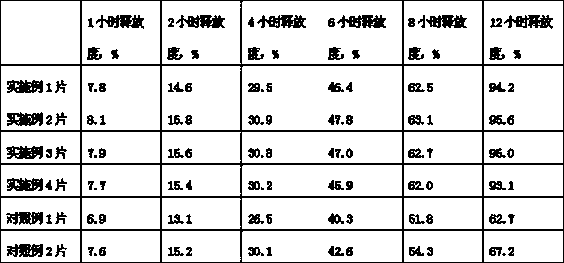
[0030] Embodiment 1, itopride hydrochloride 75g, polyethylene glycol 1000 60g, lactose 50g, mannitol 20g, micropowder silica gel 5g, povidone K30 8g. According to the preparation method described in the technical solution part of the present invention, 1000 tablet cores are prepared.
[0031] Coating layer: The mass ratio of ethyl cellulose to hypromellose is 35:10, dissolved in 90% ethanol, the weight of the coating is increased by 11%, dried, and laser-drilled with a pore size of 0.5 mm.
Embodiment 2
[0032] Example 2, Itopride hydrochloride 150g, polyethylene glycol 1000 90g, lactose 80g, mannitol 40g, micronized silica gel 9g, povidone K30 10g. According to the preparation method described in the technical solution part of the present invention, 1000 tablet cores are prepared.
[0033] The mass ratio of ethyl cellulose to hypromellose is 35:10, dissolved in 90% ethanol, and the weight of the coating is increased by 10%. Drying, laser drilling, hole diameter 0.5mm.
Embodiment 3
[0034] Example 3, Itopride hydrochloride 100g, polyethylene glycol 1000 75g, lactose 70g, mannitol 30g, micronized silica gel 7g, povidone K30 9g. According to the preparation method described in the technical solution part of the present invention, 1000 tablet cores are prepared.
[0035] The mass ratio of ethyl cellulose to hypromellose is 35:10, dissolved in 90% ethanol, and the weight gain of the coating is 10.5%. Drying, laser drilling, hole diameter 0.5mm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com