Preparation method of medicine of itopride hydrochloride for promoting gastrointestinal motility
A technology of itopride hydrochloride and gastrointestinal motility, applied in the field of medicine, can solve the problems of poor selectivity, low yield, long route, etc., and achieve the effect of novel route, high purity and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A summary is as follows: a preparation method for promoting gastrointestinal motility drug itopride hydrochloride, comprising the following steps: the first step: the synthesis of 4-(2-dimethylaminoethoxy) benzyl bromide:
[0036] Synthesize 4-(2-dimethylaminoethoxy)benzaldehyde with 2-(dimethylamino)ethyl chloride hydrochloride and p-hydroxytoluene in an inert solvent in the presence of a base, and then undergo free radical bromination Reaction synthetic target product; Concrete reaction formula is as follows:
[0037]
[0038] The second step: the synthesis of 3,4-dimethoxybenzonitrile:
[0039] Use 3,4-dimethoxybenzaldehyde as raw material to react with hydroxylamine hydrochloride, and then use thionyl chloride to dehydrate in a non-polar solvent to synthesize nitrile; the specific reaction formula is as follows:
[0040]
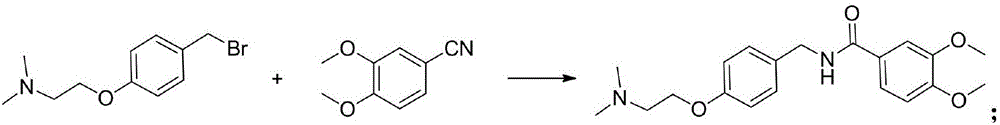
[0041] The third step: the synthesis of itopride:
[0042] Under solvent-free conditions, 4-(2-dimethylaminoethoxy)benzyl bromide and 3,4...
Embodiment 1、4
[0053] The preparation of embodiment 1,4-[2-(dimethylamino) ethoxy] toluene
[0054] Take 108g (1mol) of p-hydroxytoluene, 150g (1.1mol) of potassium carbonate, add 500mL of DMF and 50mL of isopropyl ether and stir, then add 158g (1.1mol) of 2-(dimethylamino) ethyl chloride hydrochloride and dissolve it in 200ml of DMF , add a solution of 210g of triethylamine, and stir the reaction at 80°C for 2h. After the reaction, remove the insoluble matter by filtration, recover most of the solvent from the filtrate under reduced pressure, and use 2mol·L -1 Acidify with sulfuric acid to pH to 2, add 500 mL of chloroform to extract twice, neutralize the aqueous phase with sodium hydroxide solution (w=20%) under ice water cooling, until the pH reaches 10, then extract with 500 mL of ethyl acetate, anhydrous Dry over sodium sulfate, recover ethyl acetate under reduced pressure, and distill under reduced pressure to obtain 159.3 g of colorless oil, yield: 89%.
Embodiment 2、4
[0055]The preparation of embodiment 2,4-[2-(dimethylamino) ethoxy] toluene
[0056] Take 108g (1mol) of p-hydroxytoluene, 61.7g (1.1mol) of potassium hydroxide, add 200mL of DMF and 20mL of isopropyl ether and stir, then add 158.7g (1.1mol) of (2-(dimethylamino) ethyl chloride hydrochloride Dissolve in 250DMF, add a solution of 110g of triethylamine, stir and react at 80°C for 2 hours, after the reaction, remove insoluble matter by filtration, recover most of the solvent from the filtrate under reduced pressure, and use 2mol L -1 Acidify with sulfuric acid to pH to 2, add 500 mL of chloroform to extract twice, neutralize the aqueous phase with sodium hydroxide solution (w=20%) under ice water cooling, until the pH reaches 10, then extract with 500 mL of ethyl acetate, anhydrous Dry over sodium sulfate, recover ethyl acetate under reduced pressure, and distill under reduced pressure to obtain 162.8 g of colorless oil, yield: 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com