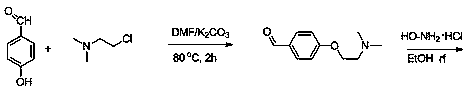
Preparation method of itopride hydrochloride
A technology of itopride hydrochloride and hydrochloride, which is applied in the field of preparation of itopride hydrochloride, can solve the problems of environmental pollution, high price, high cost, etc., and achieve the effect of short and convenient reaction route, low cost and cheap materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
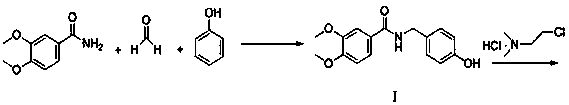
[0031] Example 1: Compound Preparation of:
[0032] Take 18.1 g (0.10 mol) of 3,4-dimethoxybenzamide, 8.1 g (containing 0.10 mol of formaldehyde) in 37% formaldehyde solution, 10.0 g of N,N-dimethylacetamide, and 18.8 g (0.20 mol) of phenol ), added 9.0 g of Hβ zeolite into a 100 mL three-neck flask, raised the temperature to 80 °C, reacted for 12 h, filtered, concentrated the filtrate to dryness, added 100.0 g of water to the obtained solid, beat at 55-65 °C for 2 h, suction filtered, and dried the solid to obtain the compound 23.0 g, yield 88.1%, HPLC purity 97.9%.
Embodiment 2
[0033] Example 2: Compounds Preparation of:
[0034] Take 90.6 g (0.50 mol) of 3,4-dimethoxybenzamide, 60.8 g of 37% formaldehyde solution (containing 0.75 mol of formaldehyde), 94.1 g (1.0 mol) of phenol, 30.0 g of silicon dioxide, and 98% concentrated sulfuric acid 15.0 g, 80.0 g of N,N-dimethylacetamide, heated to 100 °C, reacted for 10 h, filtered, concentrated the filtrate to dryness, added 500.0 g of water to the obtained solid, beat at 55-65 °C for 2 h, suction filtered, and dried the solid to obtain compound 132.2 g, yield 92.8%, HPLC purity 98.3%.
Embodiment 3
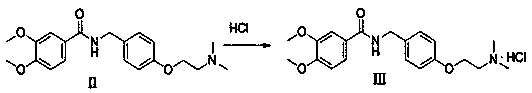
[0035] Embodiment 3: the preparation of compound II:
[0036] take compound 80.0 g (0.28 mol), 400 mL of methanol, and 31.4 g (0.56 mol) of potassium hydroxide were added to a 1000 mL three-neck flask, stirred and heated to reflux, and 40.3 g (0.28 mol), dissolved in 80.0 g of water, slowly added to the system, and monitored by thin-layer chromatography (developing agent ratio dichloromethane:methanol=10:1) until the reaction was completed. Concentrate to recover methanol, add 400.0 g of water, stir and crystallize at 10-30 °C for 3 h, filter with suction, and dry the solid to obtain 82.6 g of compound II, with a yield of 90.8% and a purity of 98.9% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com