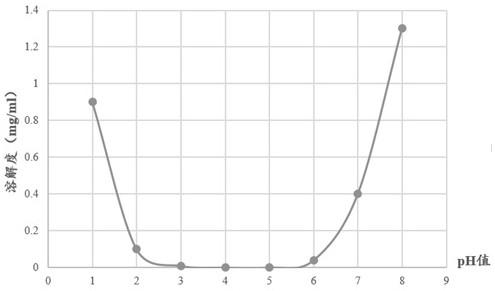
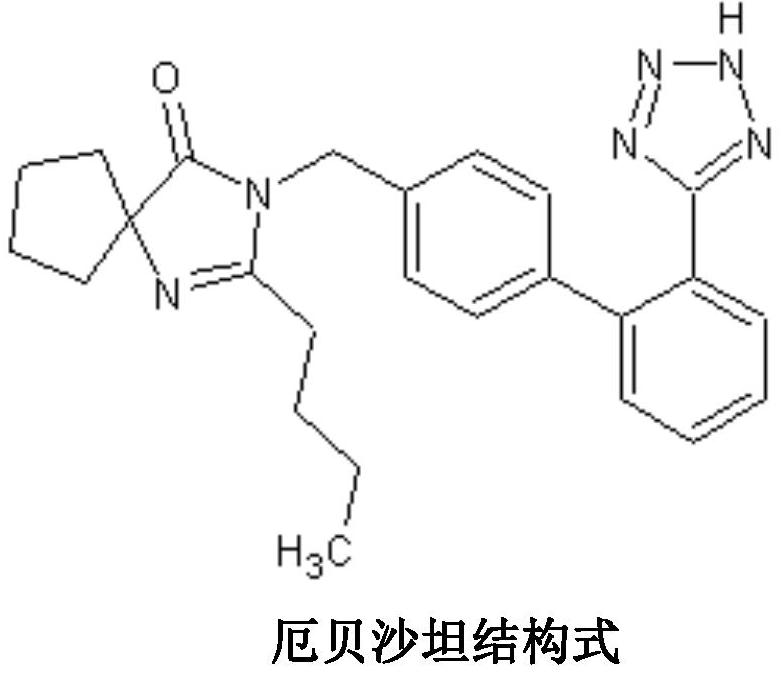
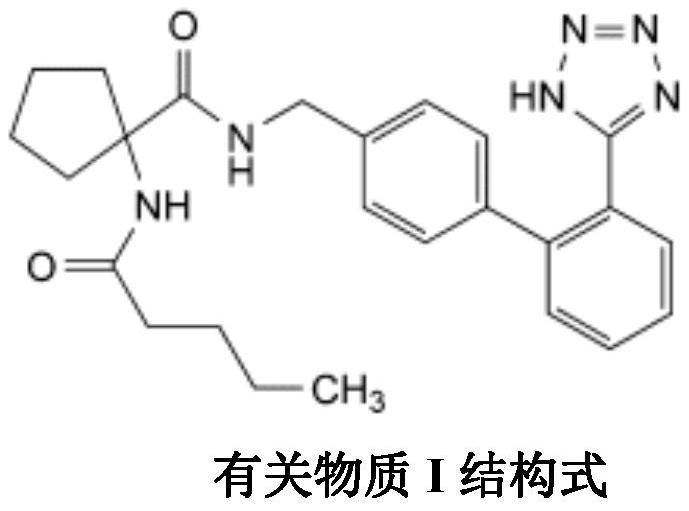
A kind of irbesartan capsule and preparation method thereof
A technology of irbesartan and capsules, which is applied in the application field in the field of consistency evaluation of generic drugs, and can solve problems such as large differences after meals and no bioequivalence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] The preparation of embodiment 1 irbesartan capsule (specification: 0.15g)
[0087] According to the prescriptions 1-4 listed in Table 8, get the materials and mark them well. The raw and auxiliary materials are passed through a 60-mesh sieve in a vibrating sieve. Weigh raw materials and excipients according to the prescription design. Receive the adhesive and prepare an adhesive solution with pure water. Put the weighed raw and auxiliary materials into the fluidized bed one-step granulator in turn, turn on the fan, and mix for 5 minutes. The prepared adhesive is top-sprayed, the air inlet temperature is controlled at 60-70°C, the material temperature is set at 50°C, dried until the water content is not higher than 2%, and the material is collected. The above-mentioned dried irbesartan granules were sized in a granulator with a sieve particle size of 2 mm. After the granulation is completed, first add the flow aid and mix in the three-dimensional mixer for 5 minutes....
Embodiment 2
[0091] Example 2 Accelerated Test Stability Research Comparison of Irbesartan Capsules (Prescription 1-2) Prepared by the Preparation Method of the Present Invention and the Original Research Product (R)
[0092] Take irbesartan capsules A and R, pack them in aluminum-plastic blisters, and place them in a constant temperature and humidity box with a temperature of 40±2°C and a humidity of RH75%±5%. , 2 months, 3 months, and 6 months at the end of sampling once respectively, check its character, content, dissolution rate and related substances, and the results are as shown in Table 9.
[0093]
[0094] The results in Table 9 show that: the irbesartan capsules (T1, T2) prepared by the present invention are placed for 6 months in a constant temperature and humidity chamber with humidity RH75%±5% at a temperature of 40 ± 2°C. No significant changes were seen in the content and content, and the related substances were not higher than the original research product, which was basi...
Embodiment 3
[0095] Example 3 Postprandial bioequivalence study of irbesartan capsules (T1, T2) prepared by the preparation method of the present invention and the original research product (R) in human body
[0096] The purpose of this test is to evaluate the test preparation T1 of irbesartan capsules (specification: 0.15g, batch number: 20171201) and the test preparation T2 of irbesartan capsules (specification: 0.15g, batch number: 20171202, manufacturer: Zhuhai Rundu Pharmaceutical Co., Ltd.) and irbesartan tablet reference preparation (trade name: Amber, specification: 0.15g, batch number: 6A248, manufacturer: Sanofi, France) Kinetic characteristics and bioequivalence.
[0097] Check-in: The subjects will be admitted to the clinical trial ward before 18:30 on the day before the administration of the first cycle, unified life management, and any food and drink other than the unified diet is prohibited. Subjects were fasted from food and water after dinner on the day before each cycle ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com