Determination method for dissolution curve of ulipristal acetate solid preparation
A technology of ulipristal acetate and solid preparations, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve problems such as high purity and manufacturer requirements, large differences in sodium lauryl sulfate, unfavorable test activities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The method for measuring the dissolution curve of ulipristal acetate tablets according to the embodiment of the present invention comprises the following steps:
[0040] 1. Paddle method
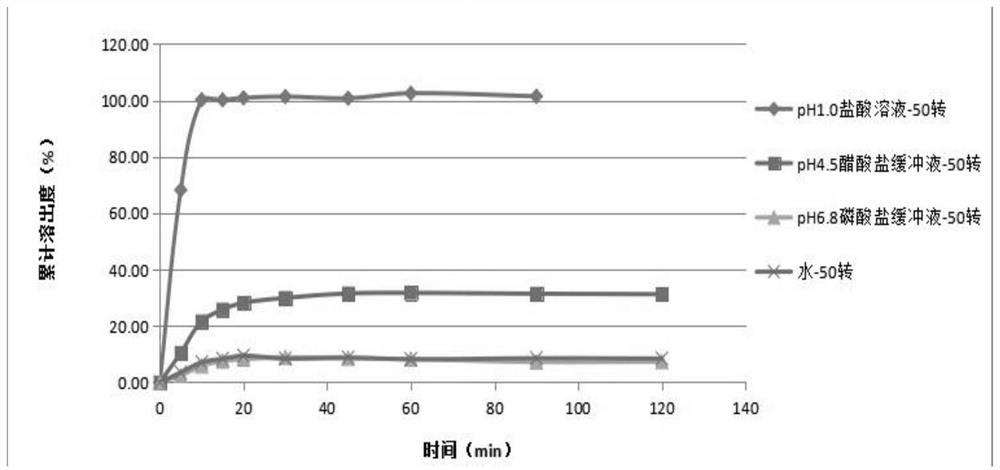
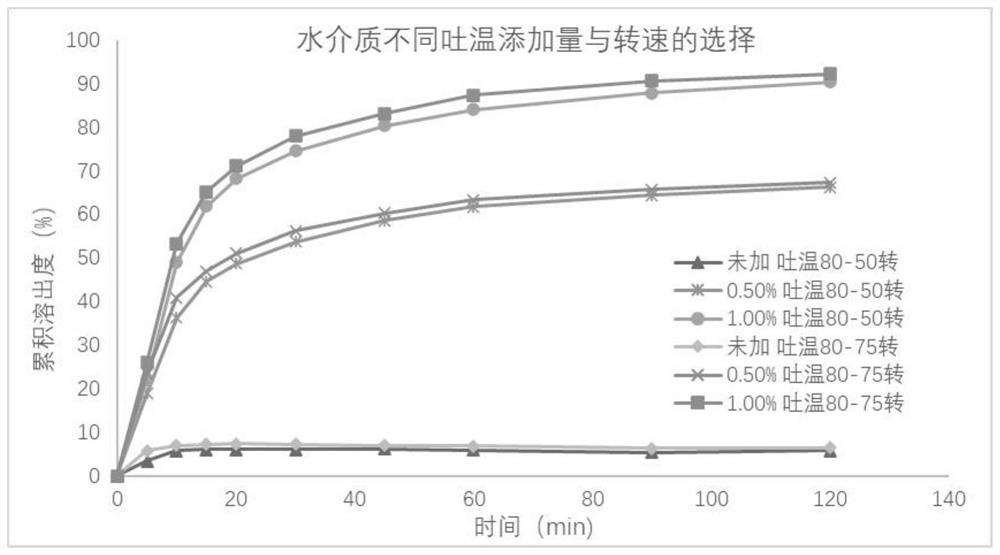
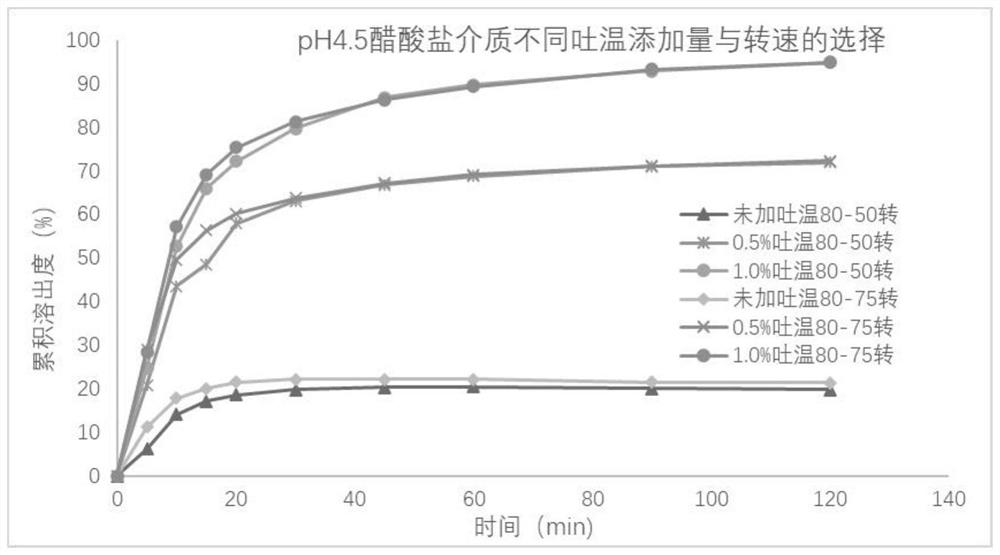
[0041] Take the reference preparation or the preparation to be tested, respectively in water, pH4.5 phosphate buffer, pH6.8 phosphate buffer 900mL, using the paddle method, 50 rpm, respectively, at 5, 10, 15, 20, At 30, 45, 60, and 90 minutes, 10 mL was sampled, filtered, and the dissolution medium at the same temperature and volume was replenished immediately. The cumulative dissolution rate of the samples was determined.
[0042] 2. Dissolution testing chromatographic conditions
[0043] Refer to the chromatographic conditions under the content determination item of ulipristal acetate tablets: with buffer solution (take 4.7 g of potassium dihydrogen phosphate, dissolve in 1000 mL of water, adjust the pH value to 7.0 with triethylamine)-organic phase (acetonitrile-tetrahydrofuran=40:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com