Method for detecting dissolution curve of simvastatin tablet
A simvastatin and curve technology, applied in the field of determination of the dissolution curve of simvastatin tablets, can solve the problems of long test time, affecting the reproducibility of experimental data, slow dissolution of simvastatin tablets, etc., and achieves good discrimination. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
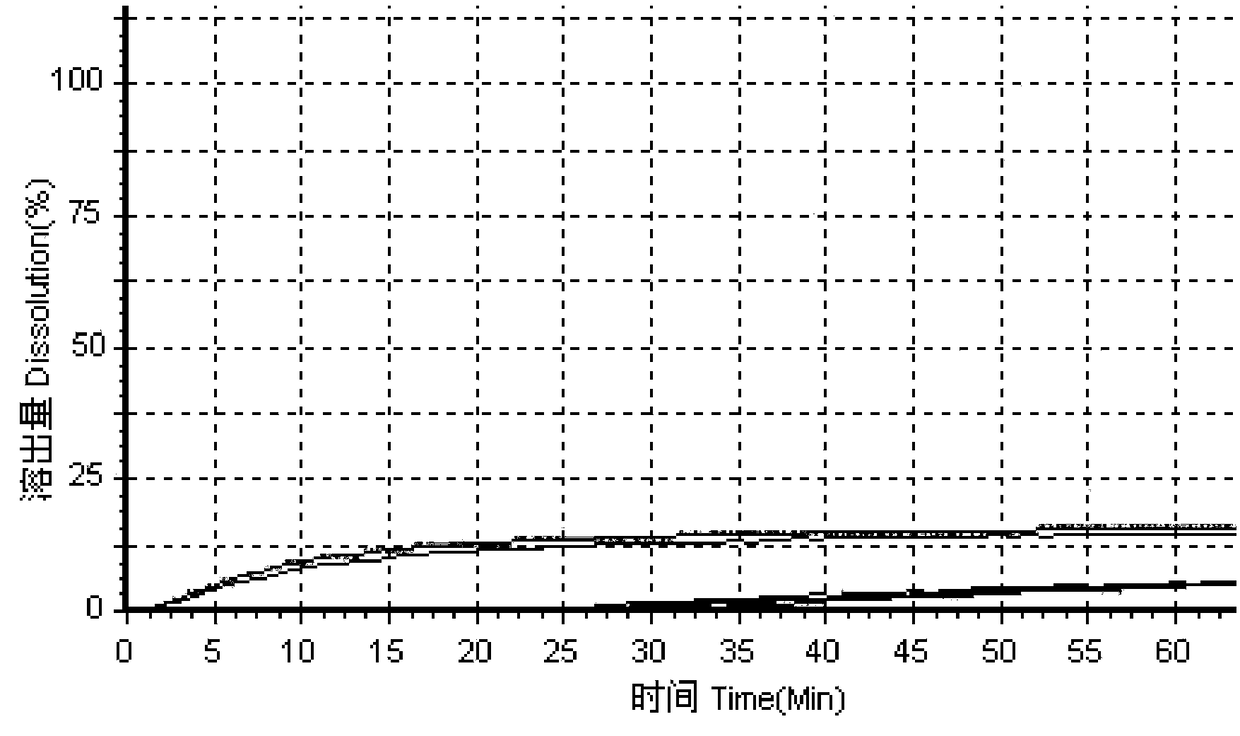
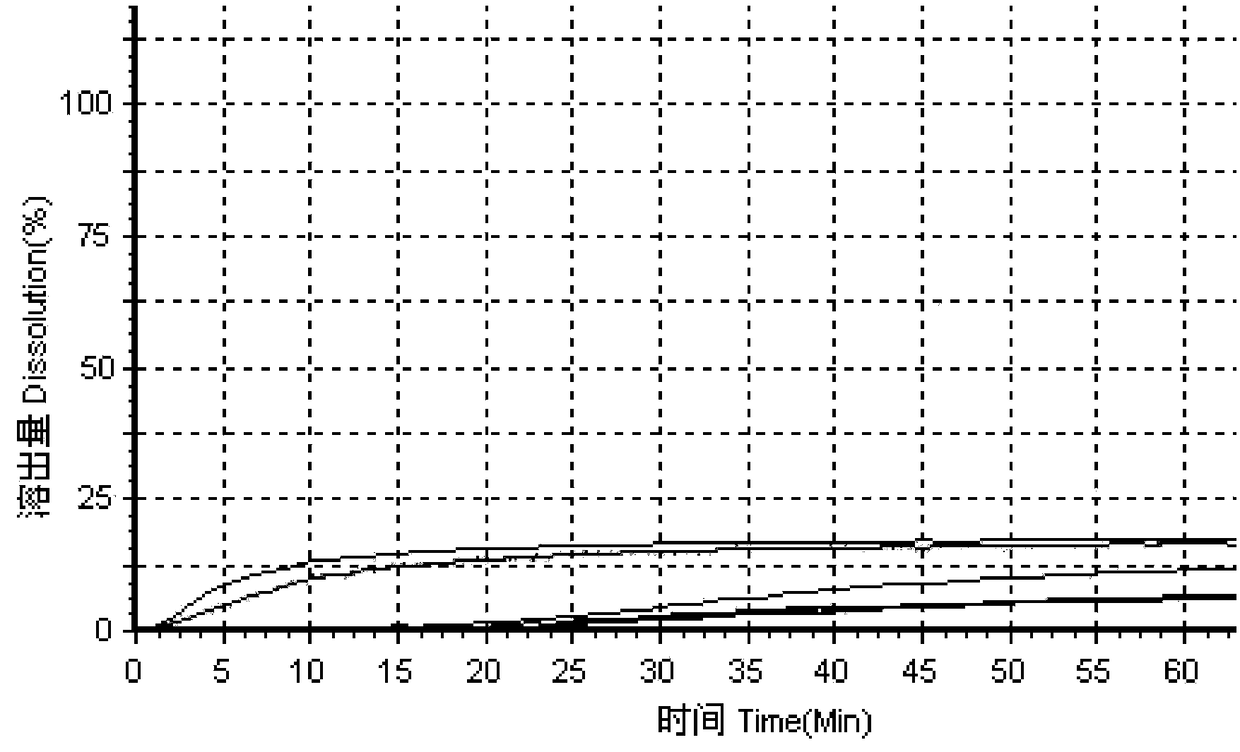
[0050] In the prior art, the method for measuring the dissolution profile of simvastatin tablets recorded in the United States Pharmacopoeia uses SDS as the dissolution medium, so in this embodiment, according to the standards of the United States Pharmacopoeia, 0.3% lauryl sulfate is used. Sodium (SDS) is used as the medium, and four kinds of dissolution media A1, B1, C1 and D1 with SDS concentration of 0.3% are configured respectively, wherein the dissolution medium A1 is 0.3% SDS aqueous solution, and the dissolution medium B1 is pH=1.2, 0.3% SDS solution, dissolution medium C1 is pH=4.0, 0.3% SDS buffer solution, dissolution medium D1 is pH=6.8, 0.3% SDS buffer solution;
[0051] Using high performance liquid chromatography, the original preparation of simvastatin tablets and three generic preparations were tested for dissolution curves using the above four dissolution media to evaluate the consistency between the three generic preparations and the original preparations; f...
Embodiment 2
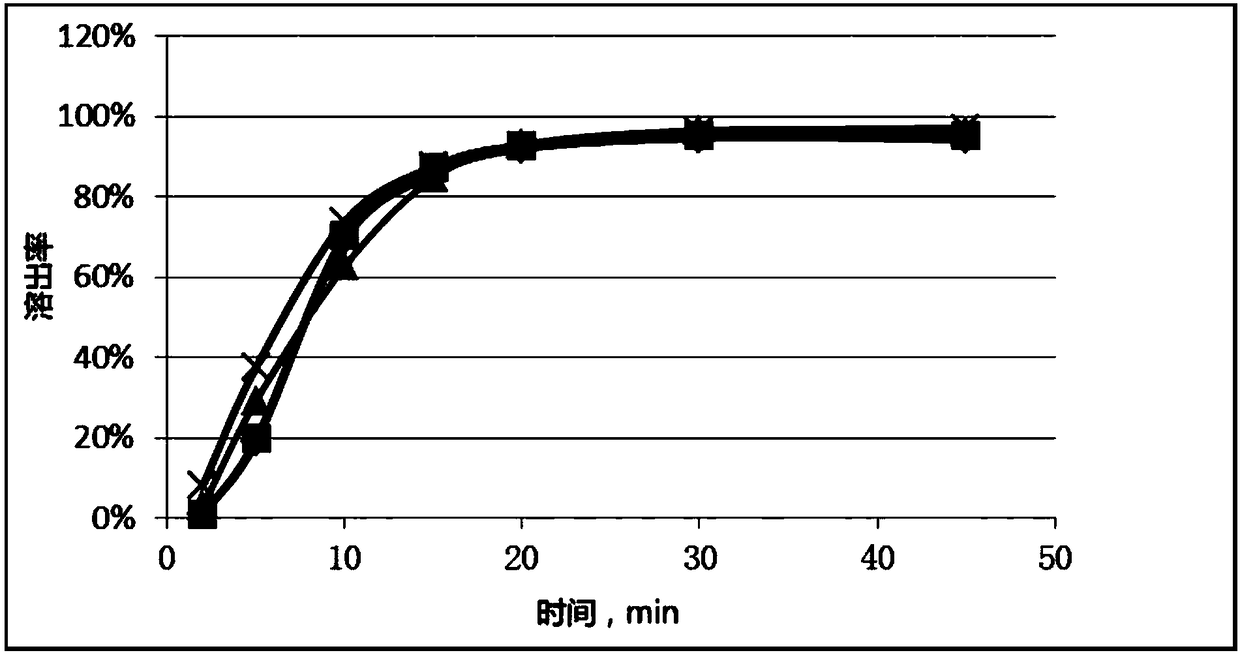
[0058] In the prior art, the method for measuring the dissolution profile of simvastatin tablets recorded in the Japanese Orange Book (Japanese Pharmacopoeia) is to take 0.3% Tween-80 as the dissolution medium, and the rotating speed is 50 rpm; In the embodiment, 0.3% Tween-80 is used as the dissolution medium, and simvastatin tablets produced by Hangzhou Merck Pharmaceutical Co., Ltd., with batch numbers of 120376 and 120374 and a specification of 20 mg, are used as the original preparation, and the Japanese Orange Book is used for the determination Methods The dissolution curve of the original preparation was determined, and compared with the standard dissolution curve recorded in the Japanese Orange Book, as shown in Figure 10 As shown, it can be seen that there is a significant difference between the dissolution curve of the original preparation prepared by the Japanese Orange Book method and the standard dissolution curve recorded in the Japanese Orange Book. The maximum ...
Embodiment 3
[0080] A method for measuring the dissolution profile of simvastatin tablets is provided in the present embodiment, comprising the steps of:
[0081] Step (1) Preparation of dissolution medium
[0082] With Tween-80 as the medium, the melting temperature of Tween-80 (or water bath temperature) is not higher than 40°C. In the optimized scheme, the melting temperature of Tween-80 (ie, water bath temperature) is controlled between 37-40°C According to this requirement, four kinds of dissolution medium A or dissolution medium B or dissolution medium C or dissolution medium D with a Tween concentration of 0.4% are configured according to this requirement, wherein,
[0083] Dissolution medium A is 0.4% Tween-80 aqueous solution, and its configuration process can be as follows: Accurately weigh 24g Tween-80 and place it in a measuring bottle, add 700-800ml water, dissolve it in a water bath at 37-40°C and transfer it to a large Container, and diluted with fresh degassed water to 600...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com