Preparation method of irbesartan capsule
A technology for irbesartan and capsules, applied in the field of preparation of irbesartan capsules, can solve problems such as difficulty in development, and achieve the effects of shortening preparation time, improving dissolution and stability, and solving inconsistent dissolution curves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The preparation of embodiment 1 irbesartan capsule (specification: 0.15g)
[0080] According to the type of material designed according to the prescription, the vibrating screen passes through a 60-mesh sieve. Weigh the raw material drug and various excipients according to the prescription design, put them in the wet granulator equipment, and mix for 5 minutes. Weigh the adhesive, and prepare an adhesive solution with 75% ethanol. Put the adhesive into the pulping device, turn on the manual pulping, and make soft materials, so that the wet granules can reach the state of being agglomerated and falling apart when touched. The soft material prepared above is placed in a rotary extrusion granulator with a screen diameter of 10 meshes, and is rotated and extruded to granulate. Set the material temperature to 50°C, and dry the irbesartan granules obtained by the above-mentioned extrusion granulation in a boiling dryer until the water content is not higher than 2%, and coll...
Embodiment 2
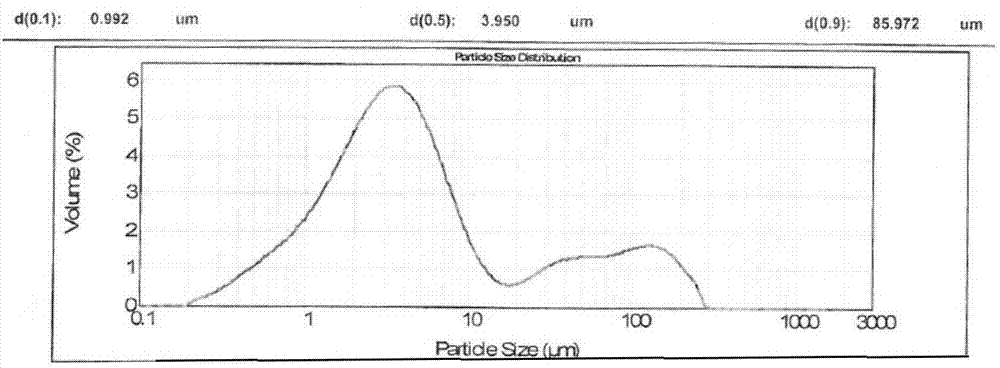
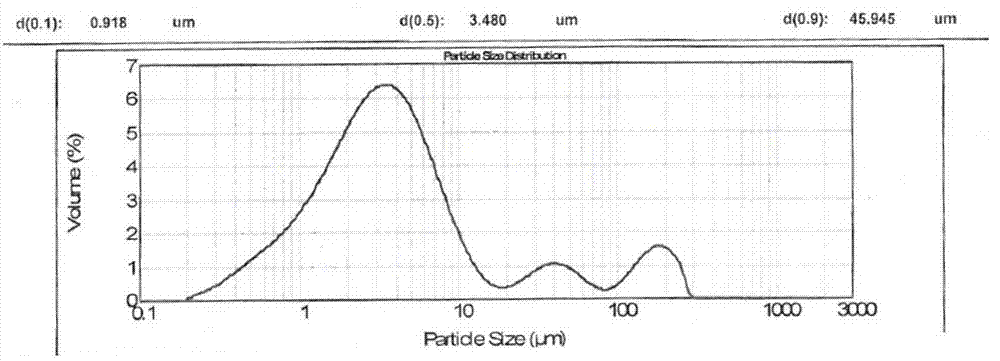
[0081] Example 2 Comparison of the in vitro dissolution curves of irbesartan capsules prepared by the present invention (prescription 1, prescription 2, prescription 3, prescription 4) and the original research product (R)
[0082] Get each 12 capsules of irbesartan capsules (prescription 1, prescription 2, prescription 3, prescription 4) prepared in Example 1 and the original research product (R), detect its hydrochloric acid solution at pH1.0, pH4.0 according to the following method The in vitro dissolution profiles of acetate buffered saline, pH 6.8 phosphate buffer and water. See Table 4 for prescriptions.
[0083] The method of table 5 irbesartan capsules in 4 different dissolution media
[0084] Dissolution medium method Rotating speed Surfactant Hydrochloric acid solution at pH 1.0 basket 75 rpm none Acetate buffer pH 4.0 basket 100 rpm 0.2% SDS Phosphate buffer at pH 6.8 basket 75 rpm none water basket 75 rpm ...
Embodiment 3
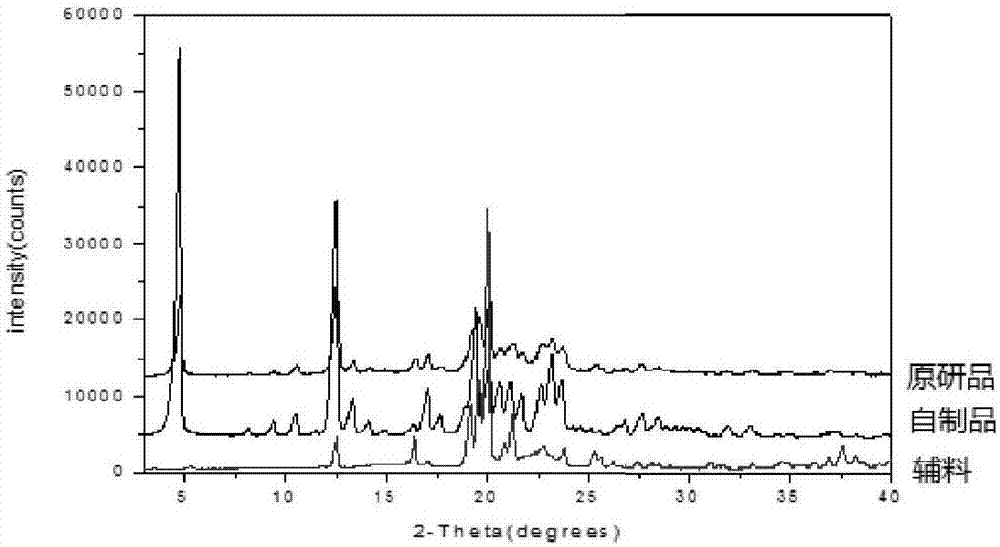
[0098] Example 3 The accelerated test stability research comparison of the irbesartan capsules (prescription 3) prepared by the preparation method of the present invention and the original research product (R)
[0099] Take irbesartan capsules A and R, pack them in aluminum-plastic blisters, and place them in a constant temperature and humidity box with a temperature of 40±2°C and a humidity of RH75%±5%. , 2 months, 3 months, and 6 months at the end of each sampling once, check its character, content, dissolution rate and related substances, the results are shown in Table 10.
[0100] The results in Table 10 show that: Rabeprazole enteric-coated capsules (A) prepared by the present invention are placed for 6 months in a constant temperature and humidity chamber with humidity RH75%±5% at a temperature of 40 ± 2°C, and the related substances, dissolution rate No significant changes were seen in the content and content, and the related substances were lower than the original rese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com