Method for determining dissolution curve of lanthanum carbonate chewable tablets
A measurement method and technology of chewable tablets, which are applied in the field of analytical chemistry, can solve the problems of ineffective distinction, poor applicability, and less use of the reciprocating cylinder method, and achieve the effects of quality and curative effect, consistency, and quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Dissolution method: paddle method, 75 rpm
[0041] Medium volume: 1000ml
[0042] Medium: 0.25N hydrochloric acid
[0043] Medium temperature: 37.0°C
[0044] Sampling time: 15, 30, 45, 60, 90, 120min
[0045] Sampling volume: take 12 parts each time, 10ml each (simultaneously supplement the dissolution medium with the same volume and the same temperature)
[0046] The test solution: 0.45μm membrane filter, take the subsequent filtrate as the test solution.
[0047] Reference substance solution: Take lanthanum ion standard solution (c=1.000mg / L) and dilute it with dissolution medium 2 times as the reference substance solution.
[0048] Get need testing solution and reference substance solution and carry out ion chromatography detection, record collection of graphs, calculate dissolution rate.
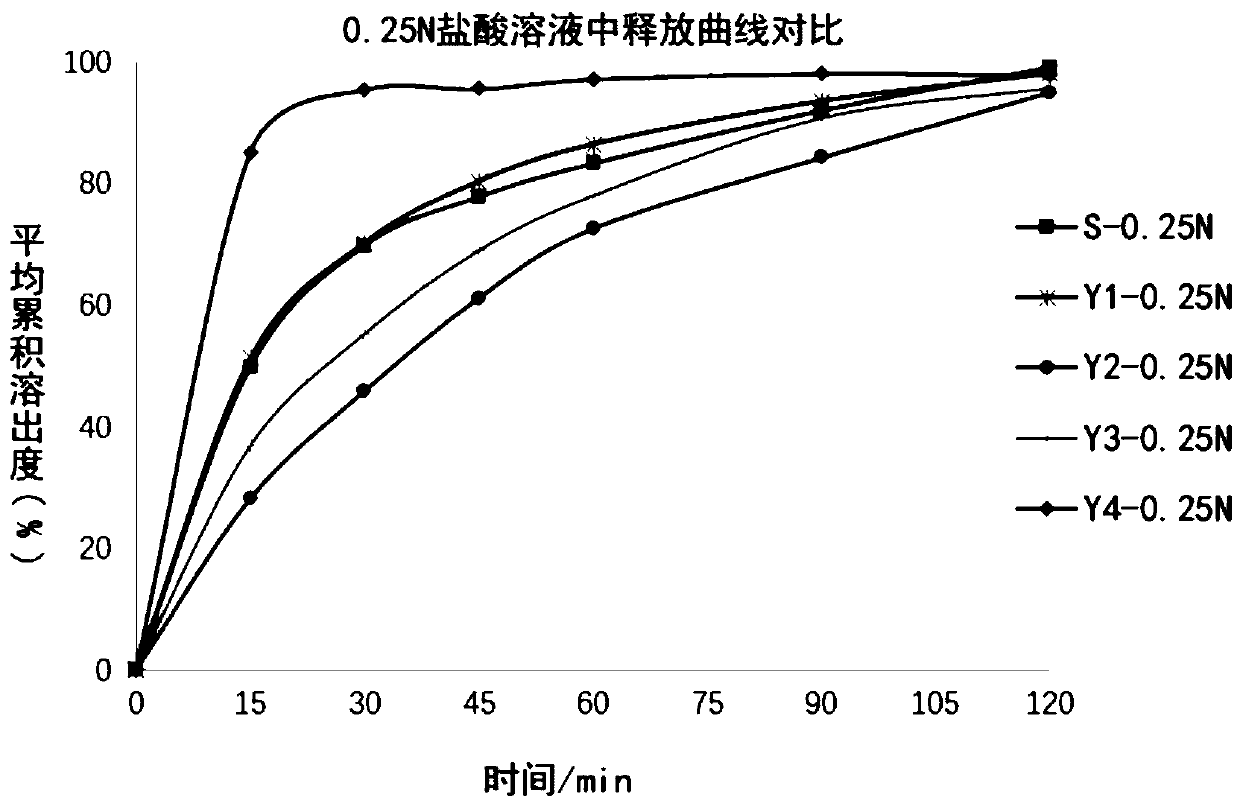
[0049] The dissolution data is shown in Table 2, and the release curve comparison is shown in figure 1 .
[0050] Table 2 Dissolution in 0.25N hydrochloric acid, 75 rpm
...
Embodiment 2
[0054] Dissolution method: paddle method
[0055] Speed: 65 rev / min
[0056] Medium volume: 1000ml
[0057] Medium: 0.25N hydrochloric acid
[0058] Medium temperature: 37.0°C
[0059] Sampling time: 15, 30, 45, 60, 90, 120min
[0060] Sampling volume: take 12 parts each time, 10ml each (simultaneously supplement the dissolution medium with the same volume and the same temperature)
[0061] The test solution: 0.45μm membrane filter, take the subsequent filtrate as the test solution.
[0062] Reference substance solution: Take lanthanum ion standard solution (c=1.000mg / L) and dilute it with dissolution medium 2 times as the reference substance solution.
[0063] Get need testing solution and reference substance solution and carry out ion chromatography detection, record collection of graphs, calculate dissolution rate.
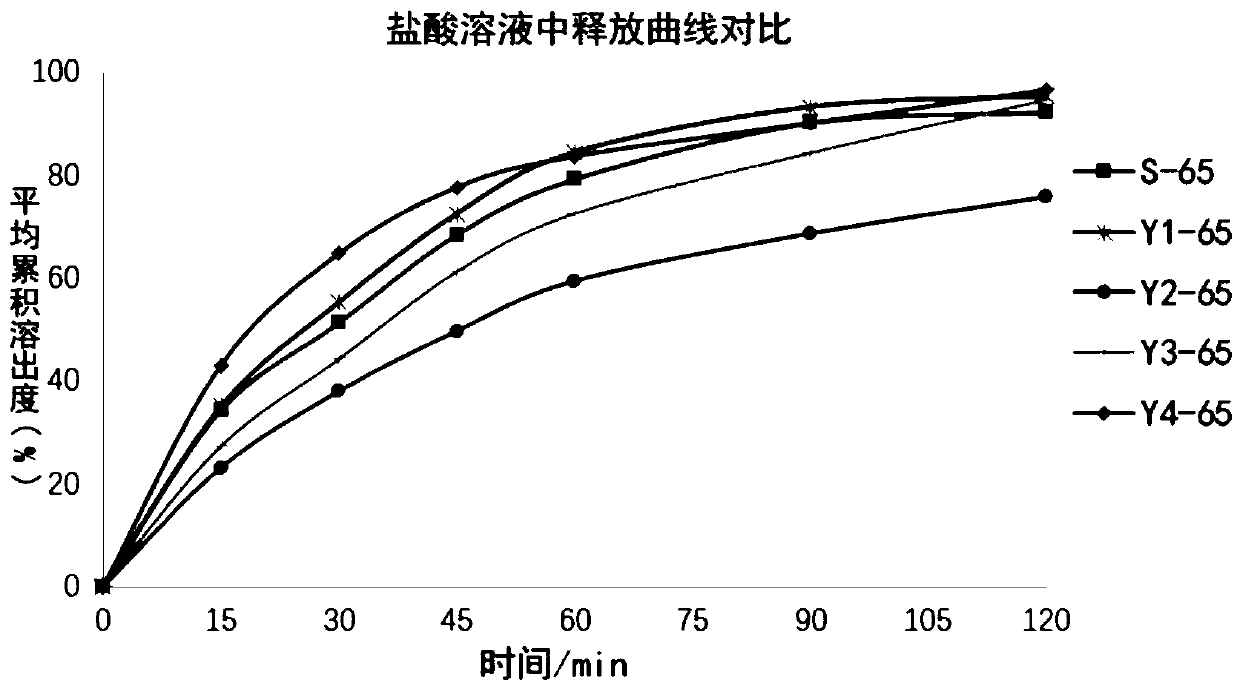
[0064] The dissolution data is shown in Table 3, and the release curve comparison is shown in figure 2 .
[0065] Table 3 Dissolution in 0.25N hydrochlo...
Embodiment 3
[0069] Dissolution method: paddle method
[0070] Speed: 50 rev / min
[0071] Medium volume: 1000ml
[0072] Medium: 0.25N hydrochloric acid
[0073] Medium temperature: 37.0°C
[0074] Sampling time: 15, 30, 45, 60, 90, 120min
[0075] Sampling volume: take 12 parts each time, 10ml each (simultaneously supplement the dissolution medium with the same volume and the same temperature)
[0076] The test solution: 0.45μm membrane filter, take the subsequent filtrate as the test solution.
[0077] Reference substance solution: Take lanthanum ion standard solution (c=1.000mg / L) and dilute it with dissolution medium 2 times as the reference substance solution.
[0078] Get need testing solution and reference substance solution and carry out ion chromatography detection, record collection of graphs, calculate dissolution rate.
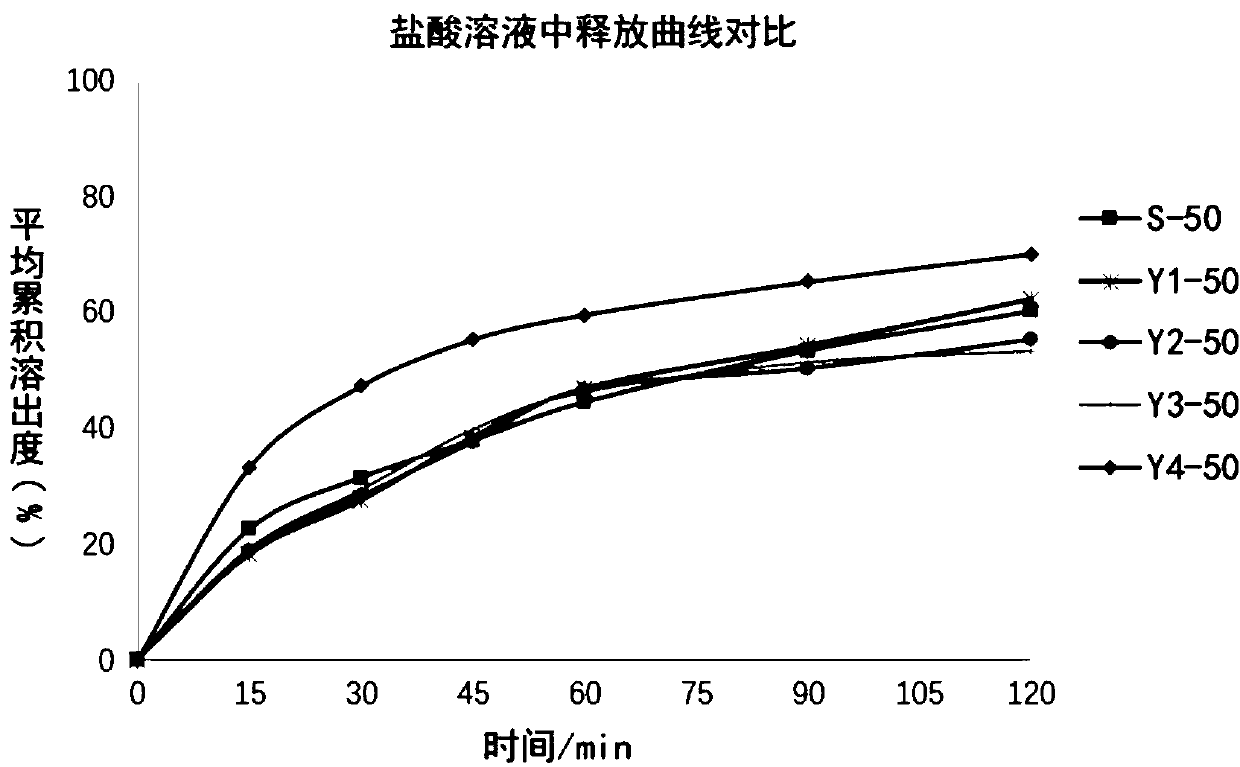
[0079] The dissolution data is shown in Table 4, and the release curve comparison is shown in image 3 .
[0080] Table 4 Dissolution in 0.25N hydrochl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com