Determination method of dissolution curve of glimepiride tablets in dissolution medium with pH of 1.2
A technology of glimepiride tablet and dissolution medium, which is applied in the field of drug analysis and can solve the problems of inability to distinguish
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
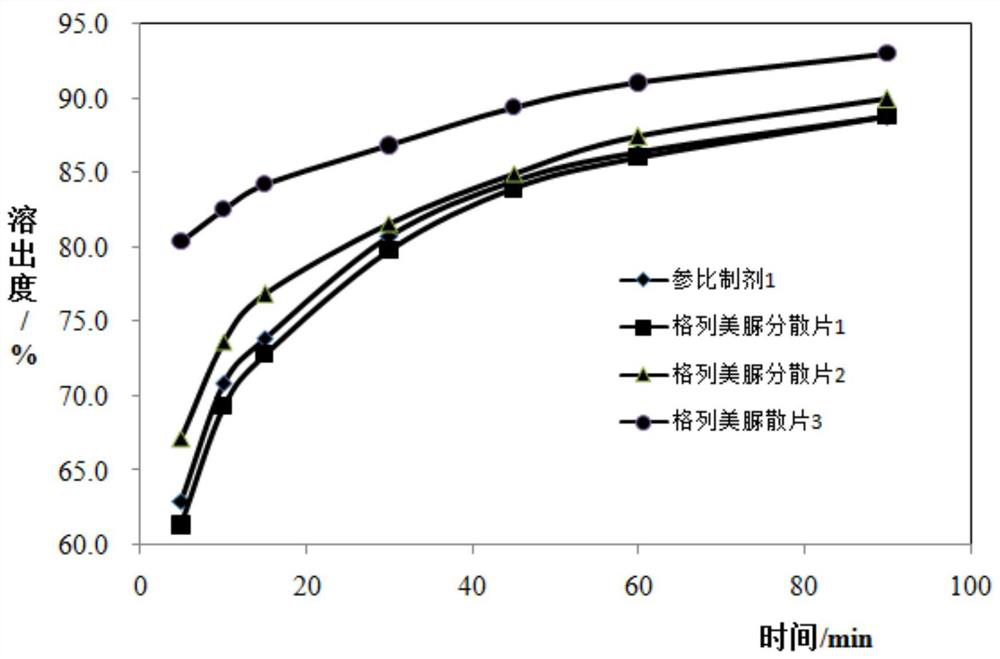
[0162] Embodiment 1: the assay method of stripping curve
[0163] (1) Reference preparation and sample to be tested:
[0164] Reference preparation: reference preparation 1, specification 1mg;
[0165] Samples to be tested: Glimepiride Dispersible Tablet 1, Glimepiride Dispersible Tablet 2, Glimepiride Dispersible Tablet 3.
[0166] (2) Detection method:
[0167] 1. Preparation of dissolution medium (both take the preparation of 1000ml as an example):
[0168] Hydrochloric acid solution of pH 1.2 containing 0.04% SDS: Measure 7.65ml of hydrochloric acid with a graduated cylinder and dilute it to 1000ml with water, then add 0.4g of sodium lauryl sulfate and stir well to obtain a hydrochloric acid solution of pH 1.2 containing 0.04% SDS .
[0169] 2. Dissolution method:
[0170] Using the paddle method, measure 900ml of dissolution medium and place them in dry dissolution cups, raise the temperature. After the temperature of the dissolution medium rises to 37°C±0.5°C, the s...
Embodiment 2
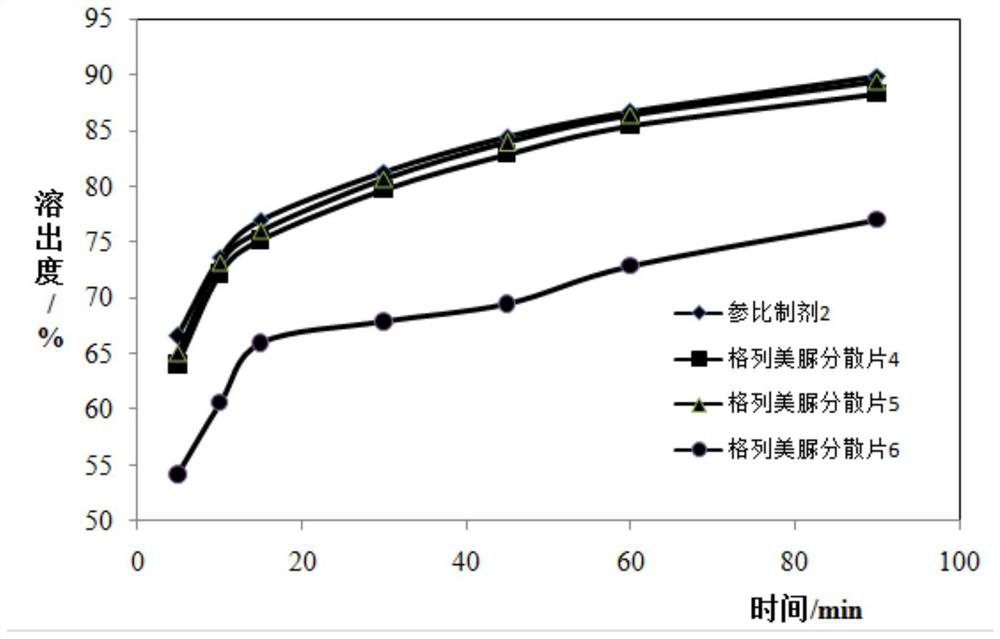
[0179] Embodiment 2: the assay method of stripping curve
[0180] (1) Reference preparation and sample to be tested:
[0181] Reference preparation: reference preparation 2, specification 2mg;
[0182] Samples to be tested: Glimepiride Dispersible Tablet 4, Glimepiride Dispersible Tablet 5, Glimepiride Dispersible Tablet 6.
[0183] (2) Detection method:
[0184] 1. Preparation of dissolution medium (both take the preparation of 1000ml as an example):
[0185] Hydrochloric acid solution of pH 1.2 containing 0.1% SDS: Use a graduated cylinder to measure 7.65ml of concentrated hydrochloric acid and dilute it to 1000ml with water, then add 1.0g of sodium lauryl sulfate and stir well to obtain hydrochloric acid of pH 1.2 containing 0.1% SDS solution.
[0186] 2. Stripping method: the rotating speed is 50 rpm, and all the other are the same as in Example 1.
[0187] 3. Dissolution testing method: the reference substance solution is as follows, and the rest are the same as in E...
Embodiment 3
[0195] Embodiment 3: the assay method of stripping curve
[0196] (1) Reference preparation and sample to be tested: with embodiment 1.
[0197] (2) Detection method:
[0198] 1. Preparation of dissolution medium (both take the preparation of 1000ml as an example):
[0199] Hydrochloric acid solution of pH 1.2 containing 0.05% SDS: use a graduated cylinder to measure 7.65ml of hydrochloric acid and dilute it to 1000ml with water, then add 0.5g of sodium lauryl sulfate and stir well to obtain a hydrochloric acid solution of pH 1.2 containing 0.05% SDS .
[0200] 2. Dissolution method: with embodiment 2.
[0201] 3. Dissolution testing method: same as in Example 1.
[0202] 4, calculation formula: with embodiment 1.
[0203] (3) Dissolution curve results:
[0204] The dissolution profile results are shown in Table 8.
[0205] Table 8: Dissolution profile of Example 3 (Glimepiride Dispersible Tablet-1mg)
[0206]
[0207]
[0208] The f of glimepiride dispersible ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com