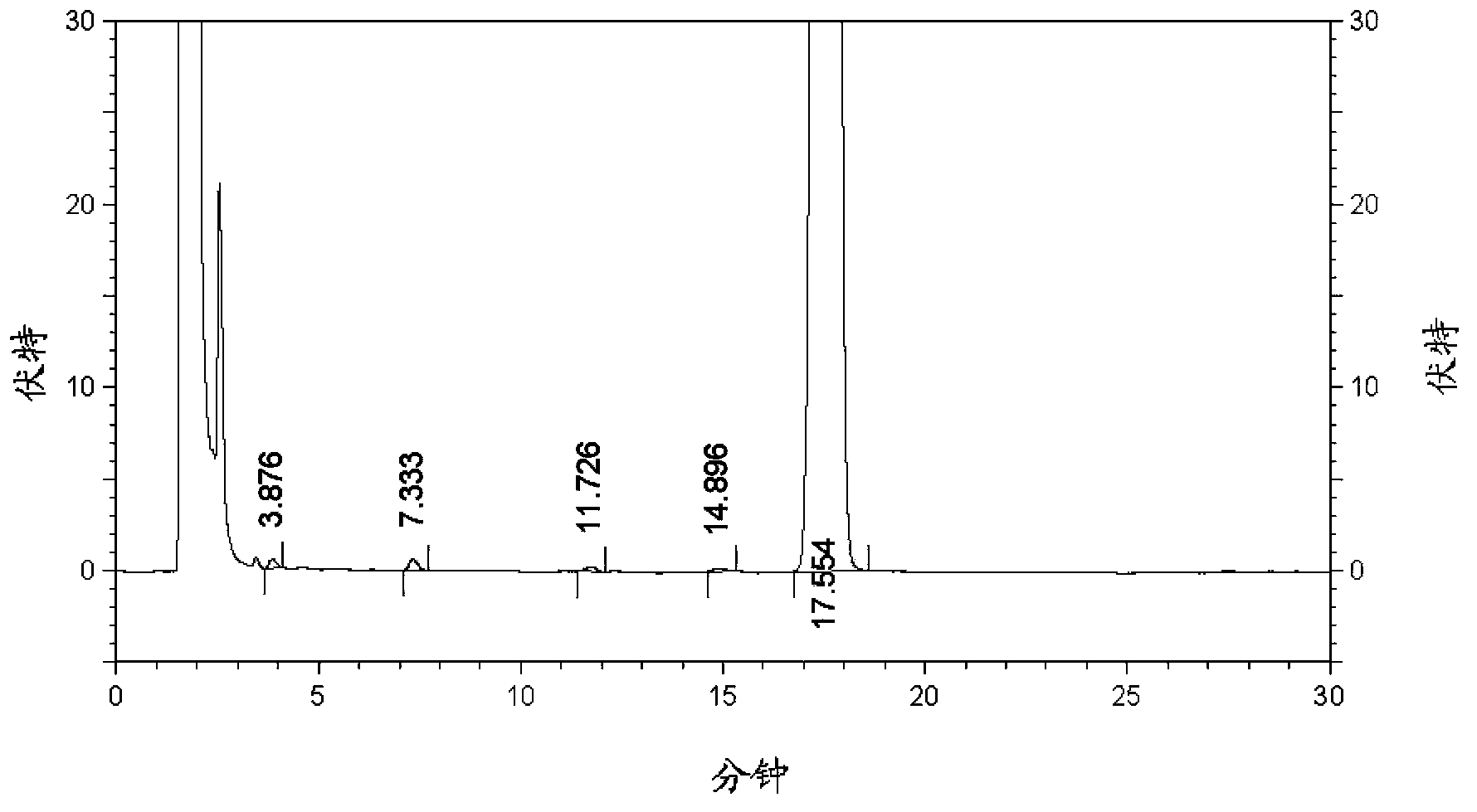
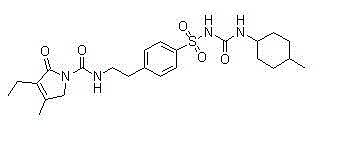
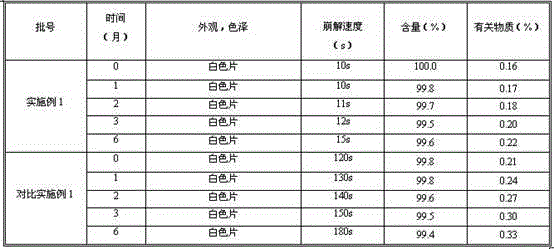
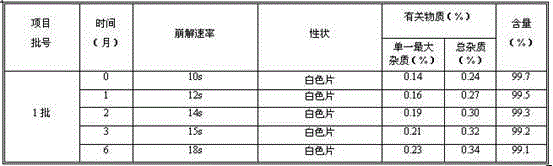
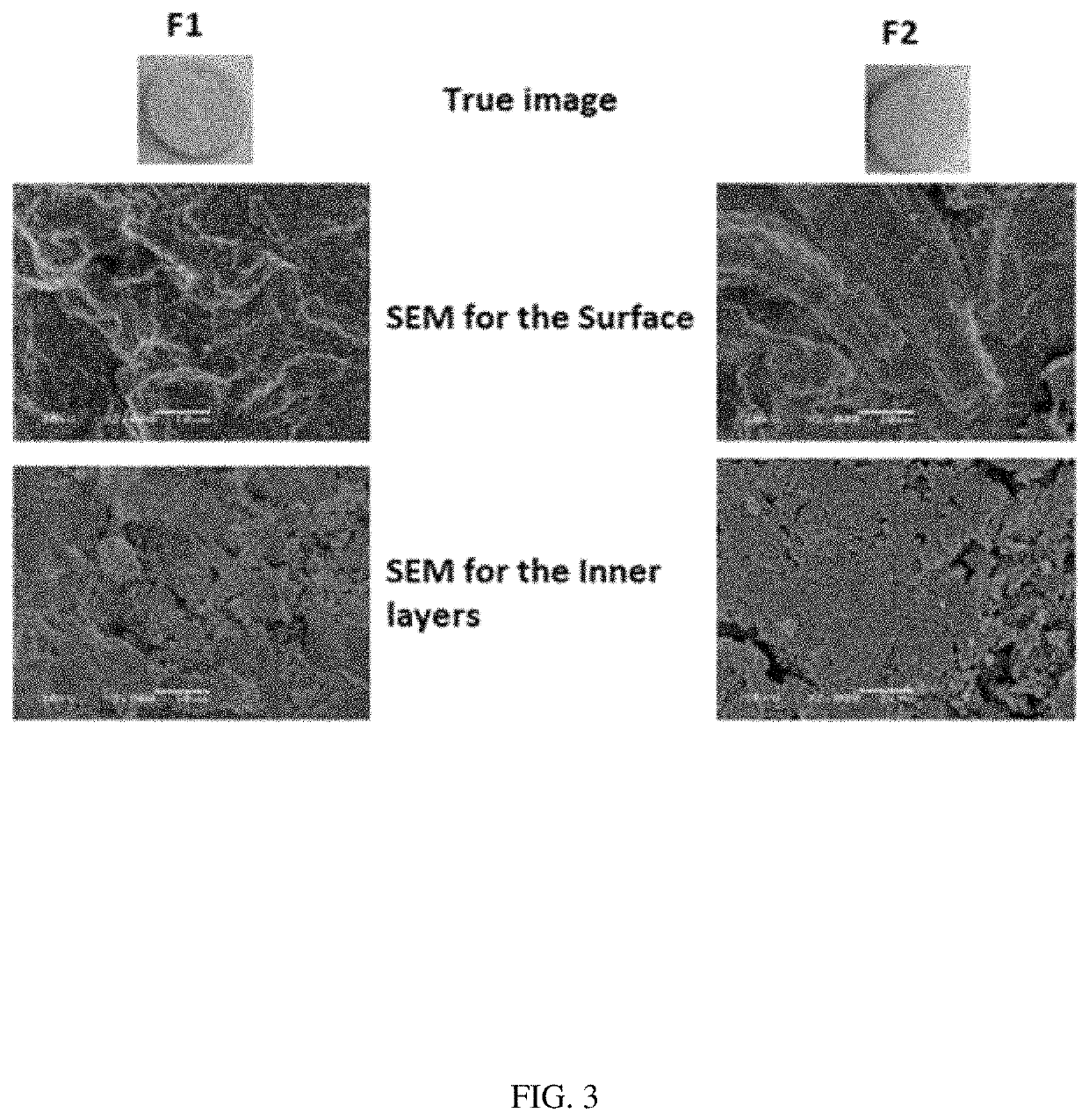
Patents
Literature
36 results about "Glimepiridum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glimepiride tablet and preparation method thereof
ActiveCN102488667BImprove hydrophilicityReduce dosageMetabolism disorderSulfonylurea active ingredientsGlimepiridumStatistical analysis
Owner:CHONGQING CONQUER PHARML
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Solid compound preparation containing metformin hydrochloride and glimepiride, preparation method and application thereof
ActiveCN103505466AHigh dissolution rateImprove stabilityMetabolism disorderSulfonylurea active ingredientsWater insolubleFiller Excipient
The invention provides a solid compound preparation containing metformin hydrochloride and glimepiride. The solid compound preparation is prepared from metformin hydrochloride granules and glimepiride granules. The metformin hydrochloride granules contain metformin hydrochloride and an adhesive, and the glimepiride granules contain glimepiride, a water soluble filler, a disintegrating agent and an adhesive. The solid compound preparation does not contain water insoluble filler. The invention also provides a preparation method and application of the solid compound preparation. Compared with common preparations and preparations adopting a water insoluble filler, the solid compound preparation provided by the invention can significantly improve the dissolution rate of glimepiride, and can effectively reduce interaction of the two main drugs, thereby reducing the increase of related impurities in a placement process. The product has stable quality and good uniformity, so that the effectiveness and safety of drug use by patients can be improved.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Glimepiride orally disintegrating medicine composition
InactiveCN102512388BHigh yieldReduce market riskMetabolism disorderSulfonylurea active ingredientsBiotechnologyGlimepiridum
The invention provides glimepiride orally disintegrating tablets. The orally disintegrating tablets are prepared by an inclusion technology, effectively solve the problems about the taste and disintegration of a main medicine, are convenient to operate, transport and store, have broad application range, and are suitable for large-scale production.
Owner:JIANGSU GAOSHIDA ELECTRIC TOOL CO LTD
Preparation method of glimepiride tablets
InactiveCN111603453AIncrease dosageIncrease dissolution rateMetabolism disorderSulfonylurea active ingredientsFluidized bed dryingCarboxymethyl starch
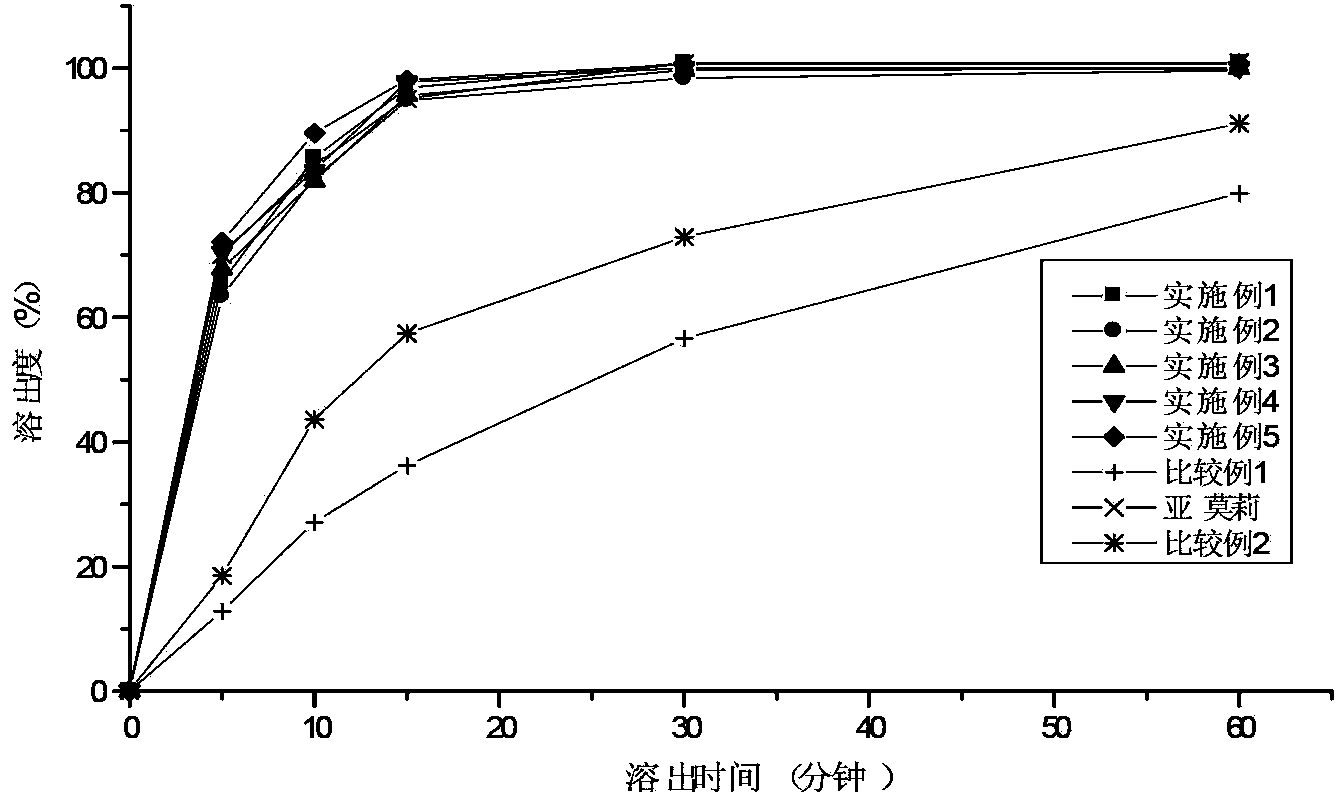
The preparation method of the glimepiride tablet comprises the following steps: S1, micronizing a glimepiride raw material medicine by adopting an airflow crushing method, and controlling the particlesize of glimepiride raw material medicine powder to be less than 10 microns; S2, weighing the glimepiride raw material medicine powder crushed in the step S1 and auxiliary materials in proportion; S3, mixing, crushing and sieving the glimepiride raw material medicine weighed in the step S2 and lactose, and adding the mixture and part of carboxymethyl starch sodium into a wet granulator for mixingto obtain premixed powder; S4, adding povidone into the wet granulator in the step S3 to prepare a soft material, granulating, and drying in a fluidized bed; and S5, adding the dried granules into the remaining sodium carboxymethyl starch, pregelatinized starch and magnesium stearate weighed in the step S2, mixing, and tabletting. The invention solves the problem of poor similarity of multiple dissolution curves of the main drug and the reference preparation in the preparation process in the prior art.
Owner:CHONGQING CONQUER PHARML
Glimepiride tablet and preparation method thereof
ActiveCN102488667AImprove hydrophilicityReduce dosageMetabolism disorderSulfonylurea active ingredientsGlimepiridumStatistical analysis
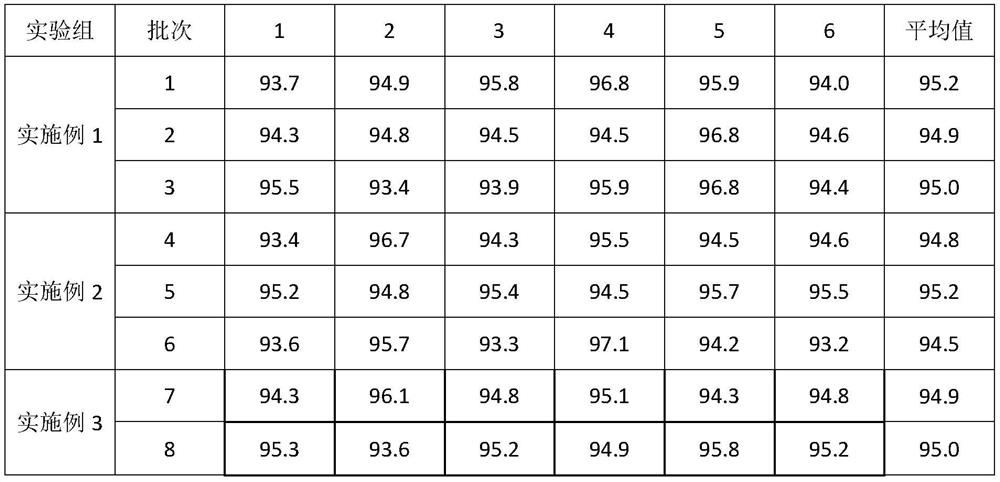
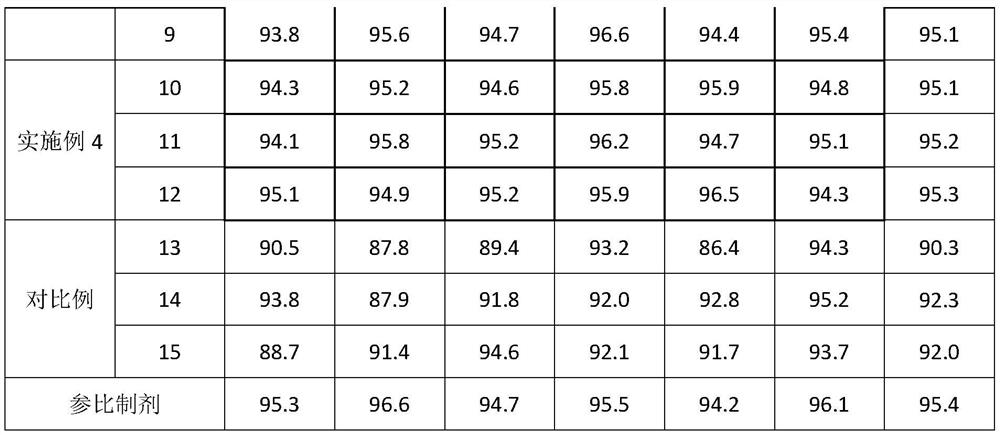
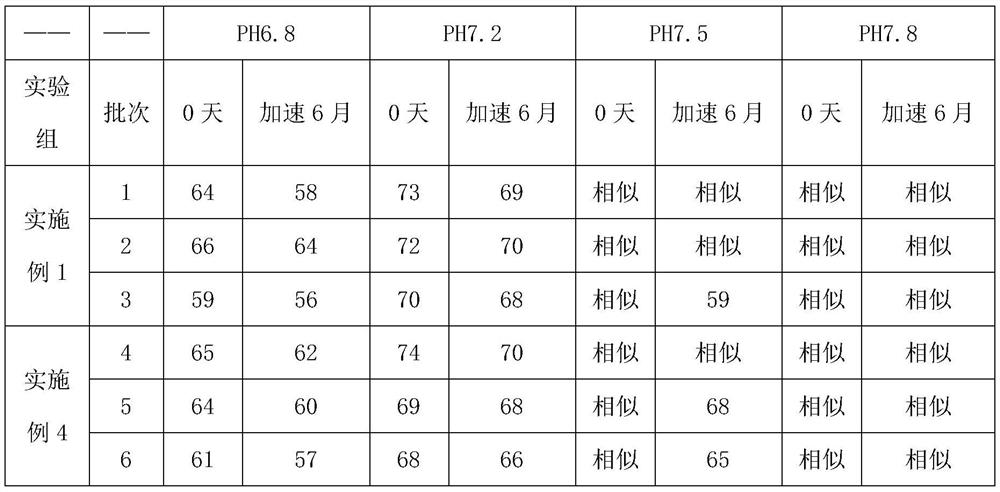
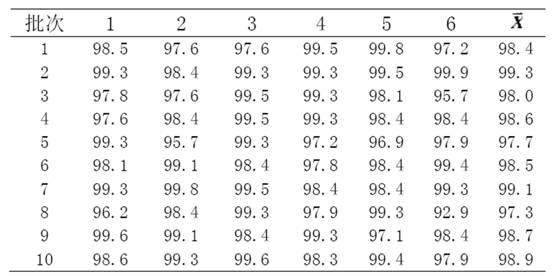
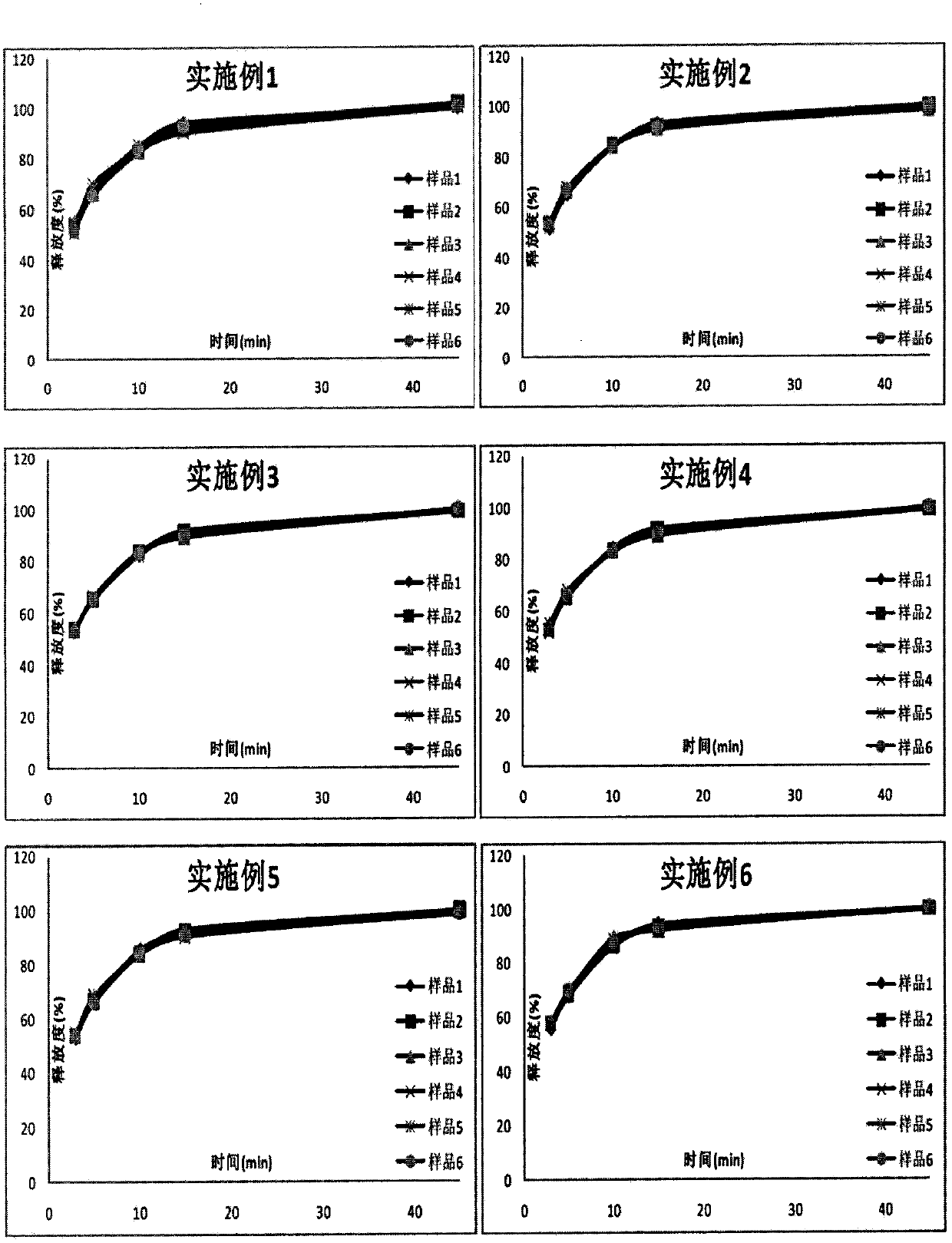
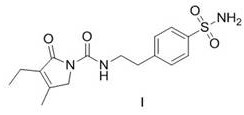
The invention discloses a Glimepiride tablet, wherein the Glimepiride tablet comprises the following components: Glimepiride tablet bulk pharmaceutical chemical, lactose, sodium starch glycolate, microcrystalline cellulose, microcrystalline silica gel and magnesium stearate at the weight ratio of (2-6):(60-90):(10-30):(10-60):(2-6):1, wherein the Glimepiride tablet bulk pharmaceutical chemical ispowder with the particle size less than 2 mu m. The invention also discloses a preparation method. With regard to Glimepiride, pharmaceuticals are smashed into particulates with the particle size less than 2mu m by an improved technology, and the hydrophilicity of the pharmaceuticals is improved; and the problem that the dissolution velocity of the pharmaceuticals is influenced is solved. Statistical analysis indicates that the average dissolution degree is above 98.5% by adopting the improved prescription and technology, and the accelerated test result for 6 months is very stable; and the research on primary pharmacokinetic parameters indicate that the preparation has the advantages of better dynamic course in human bodies and better absorption elimination properties through the checkingof statistics.
Owner:CHONGQING CONQUER PHARML
Glimepiride dispersible tablet and preparation method thereof
ActiveCN102935073ASimple preparation processHigh dissolution rateMetabolism disorderSulfonylurea active ingredientsGlimepiridumLactose
The invention discloses a glimepiride dispersible tablet. After filling agent lactose contained in the prescription is screened by a 150-mesh sieve, micronization treatment does not need to be carried out to the basic remedy, glimepiride, the dissolution rate at the time of 15 minutes can reach above 90 percent, all indexes meet the medical standard, and the stability is good. The invention also discloses a preparation method of the dispersible tablet, which has no special requirement on equipment, and is simple in operation and suitable for industrial production and application.
Owner:石药集团中诺药业(石家庄)有限公司 +1
A glimepiride tablet and a preparing method thereof
InactiveCN104027316ASmall particle sizePromote dissolutionMetabolism disorderSulfonylurea active ingredientsGlimepiridumActive agent
A glimepiride tablet and a preparing method thereof are disclosed. The preparing method includes: dissolving glimepiride in chloroform, adding a surfactant, stirring to obtain a uniform solution, drying under reduced pressure, removing the chloroform to obtain a sticky solution, adding aerosol, fully stirring, adding pharmaceutically acceptable accessory materials, uniformly mixing, and tabletting.
Owner:QINGDAO UNIV
Glimepiride dispersible tablet and preparation method thereof
ActiveCN102935073BSimple preparation processHigh dissolution rateMetabolism disorderSulfonylurea active ingredientsGlimepiridumLactose
Owner:石药集团中诺药业(石家庄)有限公司 +1
Glimepiride orally disintegrating medicine composition
InactiveCN102512388AImprove compliancePrescription Analysis Angle StabilityMetabolism disorderSulfonylurea active ingredientsBiotechnologyGlimepiridum
The invention provides glimepiride orally disintegrating tablets. The orally disintegrating tablets are prepared by an inclusion technology, effectively solve the problems about the taste and disintegration of a main medicine, are convenient to operate, transport and store, have broad application range, and are suitable for large-scale production.
Owner:JIANGSU GAOSHIDA ELECTRIC TOOL CO LTD
Analysis method for determining related substances in glimepiride intermediate by utilizing HPLC (High Performance Liquid Chromatography)
PendingCN114264765AEfficient separationHigh sensitivityComponent separationOther chemical processesGlimepiridumChemical compound
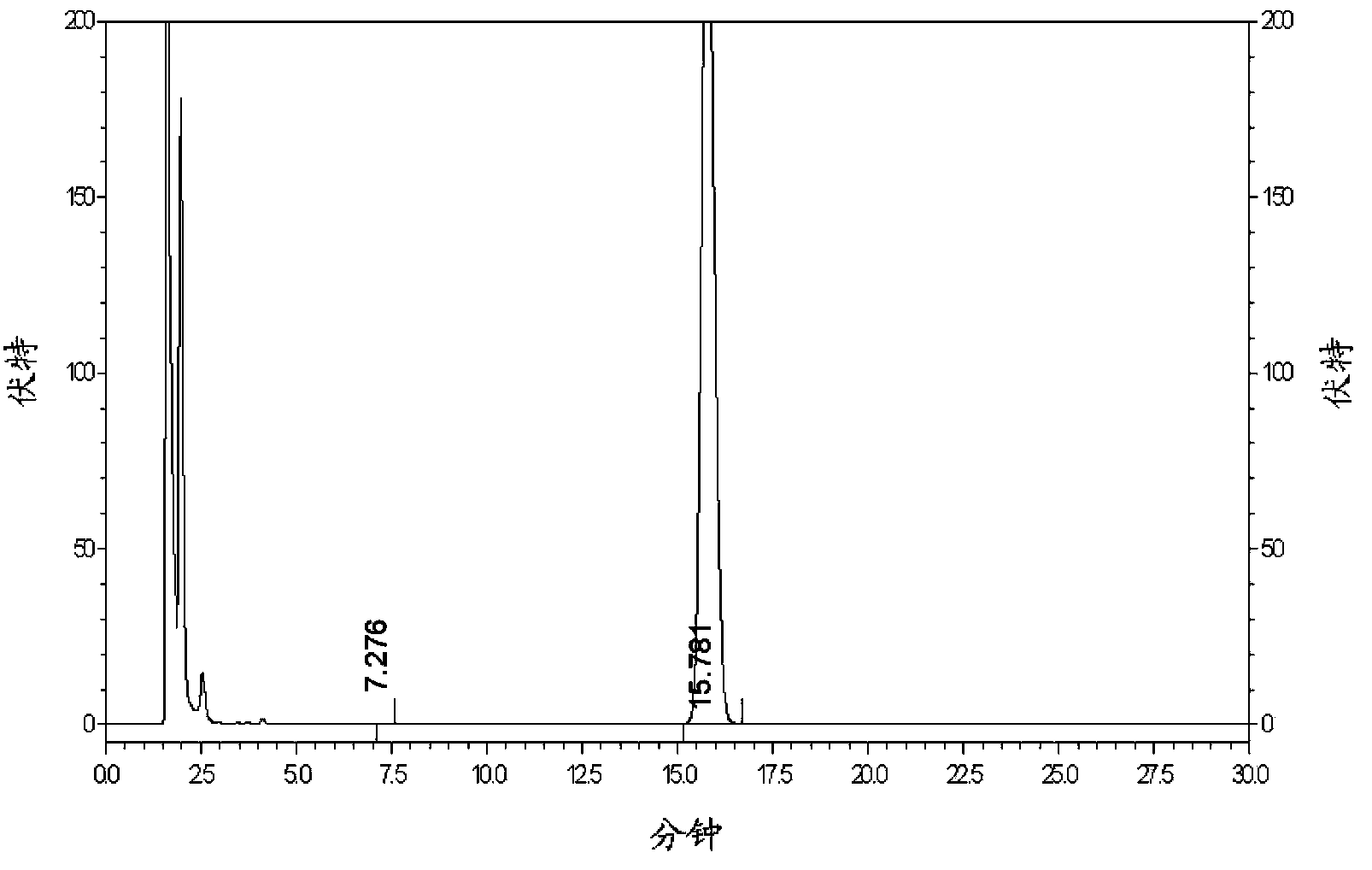
The invention discloses an analysis method for determining related substances in a glimepiride intermediate by utilizing HPLC (High Performance Liquid Chromatography), and particularly relates to an HPLC analysis method for the glimepiride intermediate and four impurities in the glimepiride intermediate. According to the present invention, with the chromatographic column type and specification, the detection wavelength, the mobile phase type, the mobile phase ratio, the operation time and the like, the impurities in the glimepiride intermediate can be rapidly, simply, accurately and efficiently eluted, separated and quantitatively detected, the complete separation between the impurity peak and the main peak and the complete separation between the impurities and the impurity peaks can be achieved, and the optimal detection result can be achieved; and the analysis method is convenient to operate, short in running time, high in specificity, sensitivity and stability, has a better linear curve in a low concentration range, has higher repeatability and accuracy, is not influenced by personnel and instruments, is stable and reliable, and provides a basis for research, development and quality detection of the compounds.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
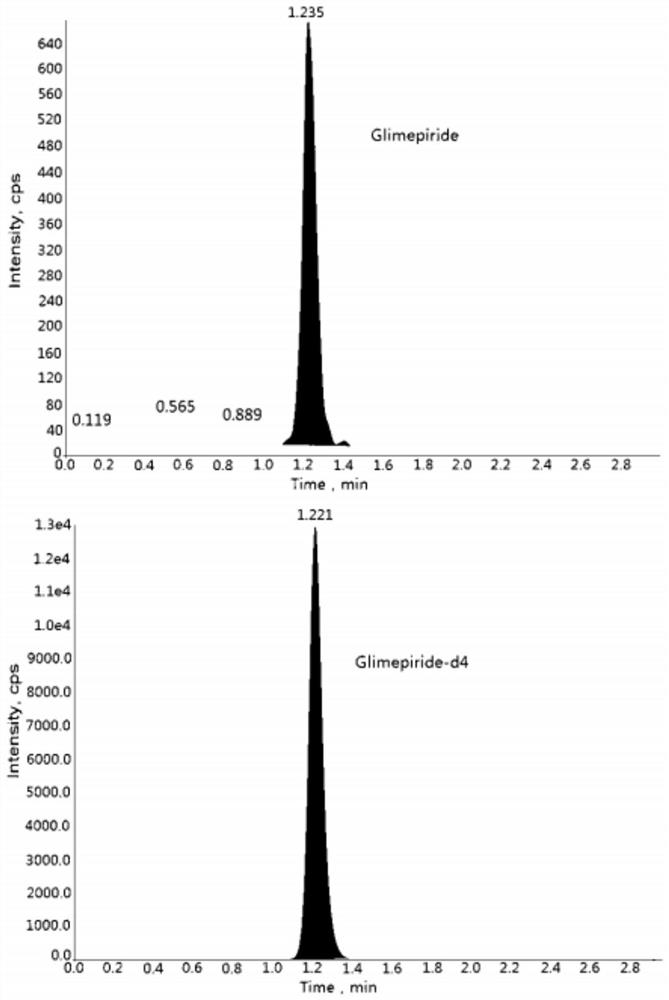
Method for determining concentration of glimepiride in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927304AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
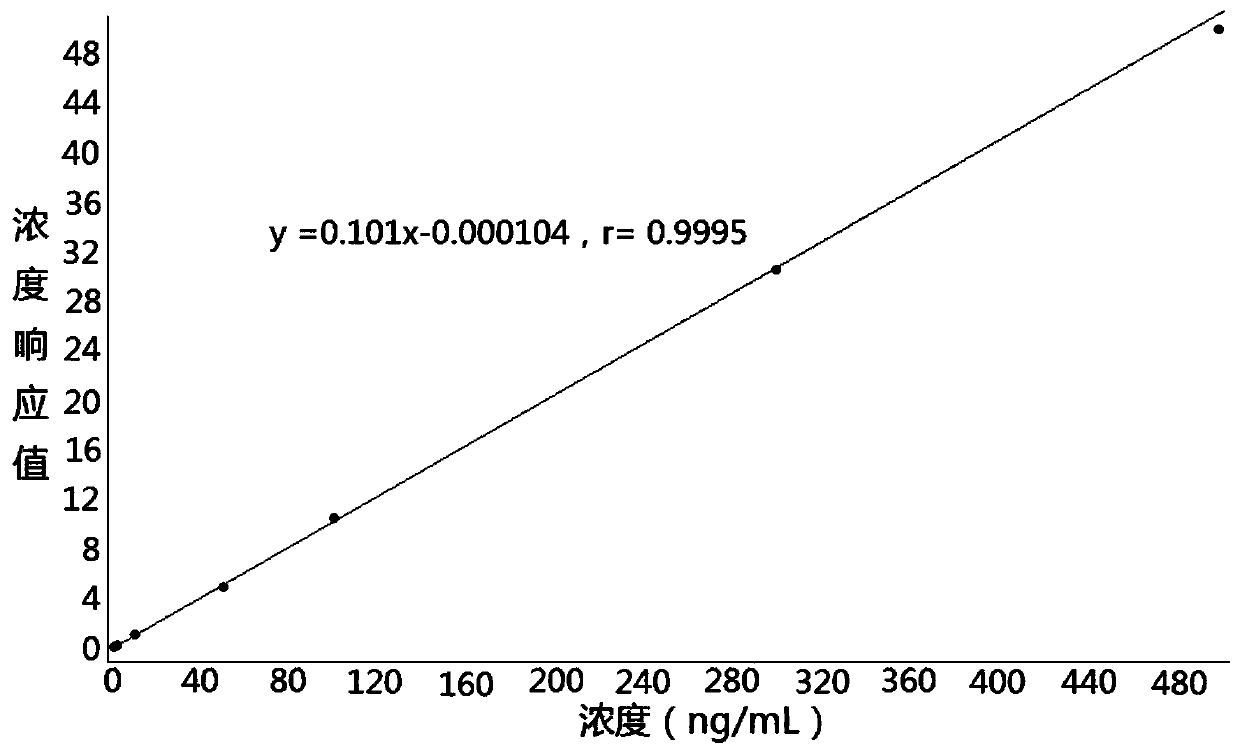
The invention discloses a method for determining the concentration of glimepiride in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: taking a sample to be determined, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by achromatographic column, and performing detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of glimepiride; the linear range of a plasma standard curve of the method is 0.5-500 ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of glimepiride in plasma.
Owner:徐州立兴佳正医药科技有限公司
Preparation method of high-purity glimepiride
ActiveCN110885306AHigh yieldWell researched and controlledOrganic chemistry methodsGlimepiridumEthyl group
The invention discloses a preparation method of high-purity glimepiride. The preparation method comprises the following specific steps: 1, adding 1-[4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)-ethyl]-benzenesulfonamide and potassium carbonate into a mixed solvent, and raising the temperature to dissolve the added substances; and 2, carrying out cooling crystallization on a solution obtained in step 1, and filtering the cooled solution to obtain 1-[4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)-ethyl]-benzenesulfonyl]-3-(trans-4-methylcyclohexyl)-urea. The glimepiride obtained by the preparation method has a purity of above 99.9% and a high yield, impurity research is sufficient and controllable, the crystal form is correct, and the preparation product quality is good,so the use requirements of people are met.
Owner:江苏海悦康医药科技有限公司
Controlled-release preparation containing metformin hydrochloride and glimepiride and preparation method thereof
ActiveCN105878256BImprove release uniformityImproved controlled releaseMetabolism disorderSulfonylurea active ingredientsGlimepiridumImmediate release
The invention relates to the field of pharmaceutical preparations, and in particular discloses a compound controlled-release preparation of metformin hydrochloride and glimepiride and a preparation method of the controlled-release preparation. The controlled-release preparation disclosed by the invention consists of an internal controlled-release tablet which contains the metformin hydrochloride and an external drug-loaded coating which contains the glimepiride, wherein the internal controlled-release tablet which contains the metformin hydrochloride comprises a tablet core, an insoluble semipermeable coating film and drug-release small pores. The controlled-release preparation disclosed by the invention can significantly improve the linearity and the uniformity of drug release, the process (the preparation method) is simple and easy to implement, and the tablet is better in compressibility; and the glimepiride, without micronizing treatment or other special treatment, is excellent in dissolving effect.
Owner:HEFEI LIFEON PHARMA
Primer for detecting sulfonylureas receptor 1 gene polymorphism sites, the application thereof and kits thereof
ActiveCN101445824AAvoid blindnessEasy to operateMicrobiological testing/measurementGlimepirideGlisolamide
The invention provides an auele specific primer for detecting SUR1 gene T1369G polymorphism sites (rs757110) genotype by a PCR method, the application of the auele specific primer in preparing a reagent for detecting SUR1 gene T1369G polymorphism sites by a PCR augmentation biological sample, a SUR1 gene T1369G polymorphism site detecting kit containing auele specific primers and the application of the detecting kit in predicting drug effect of sulfonylureas. The medicines of the sulfonylureas are selected from glibenclamide, glibornuride, glycyclamide, glyhexamide, glimepiride, glypinamide, glisamuride, glisentide, glisolamide, glyoctamide, gliclazide, glipizide and gliquidone. The selective preference is gliclazide or glimepiride.
Owner:深圳泰乐德医疗有限公司
A kind of glimepiride tablet and preparation method thereof
ActiveCN106913545BPromote dissolutionSimple preparation processMetabolism disorderSulfonylurea active ingredientsAcetic acidGlimepiridum
The invention relates to a glimepiride tablet and a preparation method thereof. The tablet is prepared by the following method: dissolving glimepiride in a sodium hydroxide solution, adding mesoporous silicon dioxide, stirring evenly, and stirring Add glacial acetic acid to this solution, the glimepiride and mesoporous silica complex precipitates, filter, dry, then mix with fillers and disintegrants, granulate, dry, add lubricant to the dry granules, Formed into tablets. Compared with the prior art, the tablet prepared by the invention dissolves rapidly in water, has a simple preparation process and is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Primer, application and kit for detecting polymorphic site of sulfonylurea receptor 1 gene
ActiveCN101445824BAvoid blindnessEasy to operateMicrobiological testing/measurementSpecific testSulfonylurea
The invention provides a specific primer for detecting the T1369G polymorphic site (rs757110) genotype of the SUR1 gene by a PCR method, and the specific primer is used in the preparation of reagents for detecting the T1369G polymorphic site of the SUR1 gene by PCR amplification of biological samples The application in the invention, the SUR1 gene T1369G polymorphic site detection kit containing specific primers and the application of the detection kit to predict the effect of sulfonylurea drugs. Sulfonylurea drugs are selected from glibenclamide, glibenclamide, glibenclamide, glibenclamide, glimepiride, glibenclamide, glibenclamide, glibenclamide, glibenclamide , glipizide, gliclazide, glipizide, and gliquidone, preferably gliclazide or glimepiride.
Owner:深圳泰乐德医疗有限公司
Glimepiride tablet and preparation method thereof
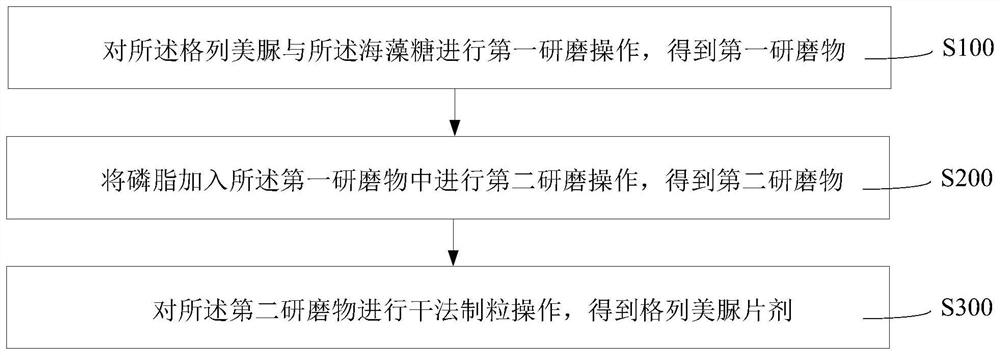
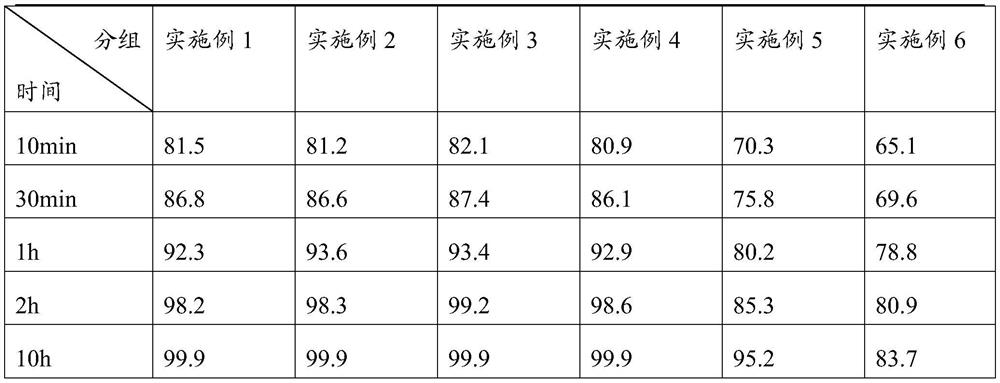
PendingCN114306253AGood hygroscopicityLow hygroscopicityMetabolism disorderSulfonylurea active ingredientsPhospholipinGlimepiridum
The invention provides a glimepiride tablet and a preparation method thereof. The glimepiride tablet comprises glimepiride, trehalose and phospholipid which are mixed and ground. The glimepiride tablet disclosed by the invention is relatively good in dissolution rate and relatively high in qualified rate of preparation.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD +1
Glimepiride dispersible tablet composition and preparation method thereof
ActiveCN112618499ABeneficial technical effectQuality improvementDispersion deliveryMetabolism disorderGlimepiridumPyrrolidinones
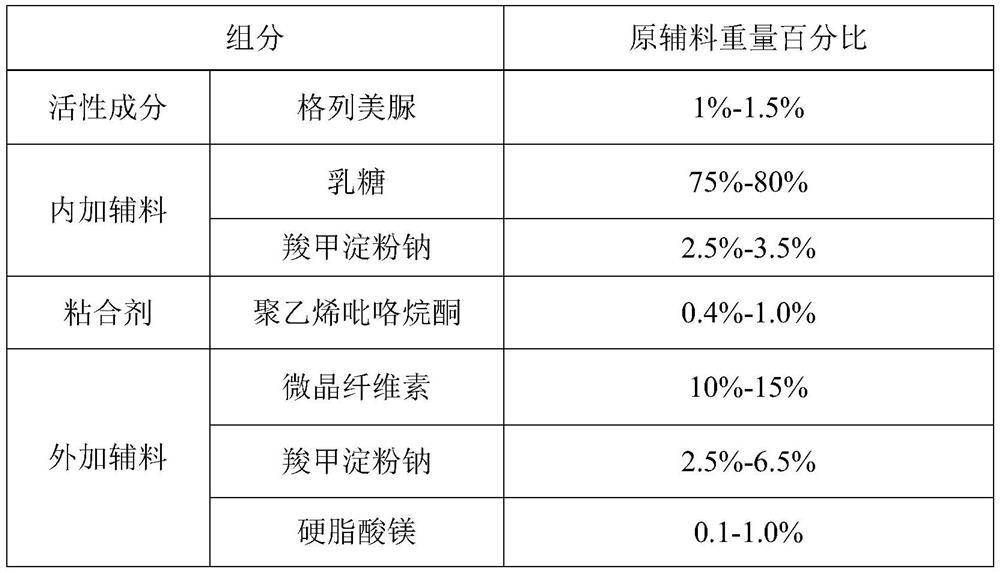
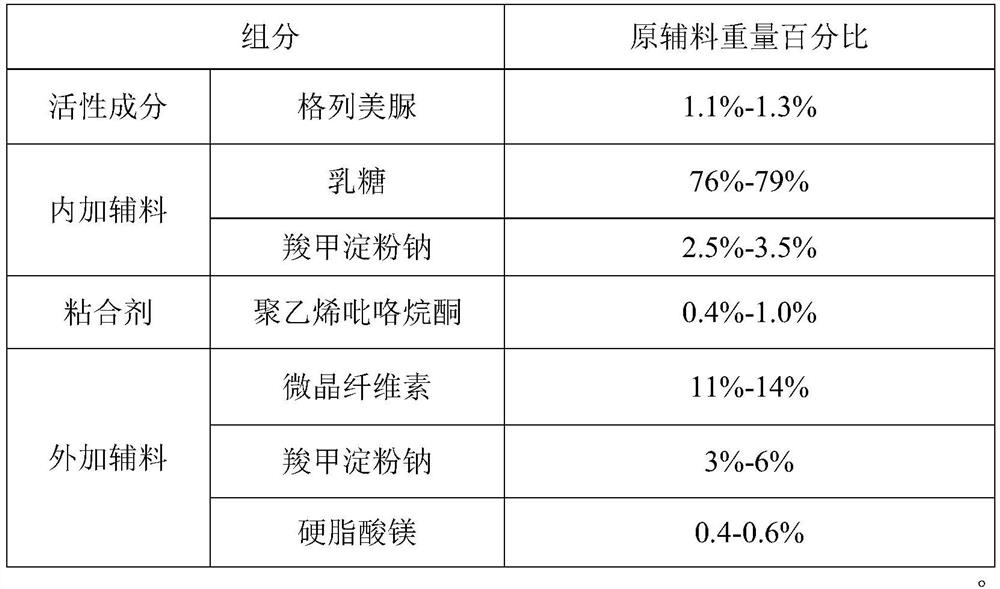
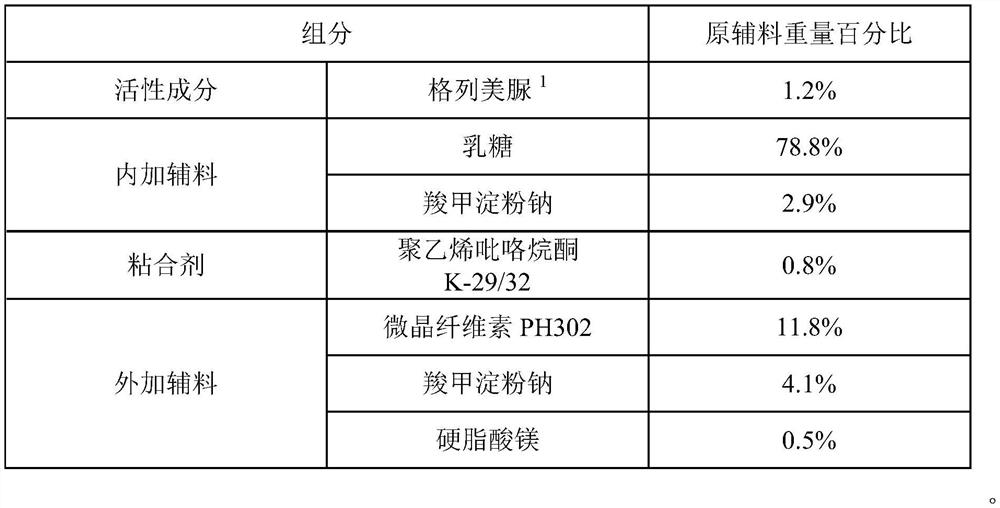
The invention provides a glimepiride dispersible tablet and a preparation method thereof. The glimepiride dispersible tablet comprises the following components in percentage by weight of 1%-1.5% of glimepiride, 75%-80% of lactose, 5%-10% of carboxymethyl starch sodium, 0.4%-1.0% of polyvinylpyrrolidone, 10%-15% of microcrystalline cellulose and 0.1%-1.0% of magnesium stearate. The glimepiride dispersible tablet prepared by the invention has dissolution rates of more than 85% in dissolution media with pH values of 1.2 and 7.8, and is high in dissolution rate and stable in clinical curative effect.
Owner:CSPC OUYI PHARM CO LTD
Determination method for related substances in glimepiride bulk drug
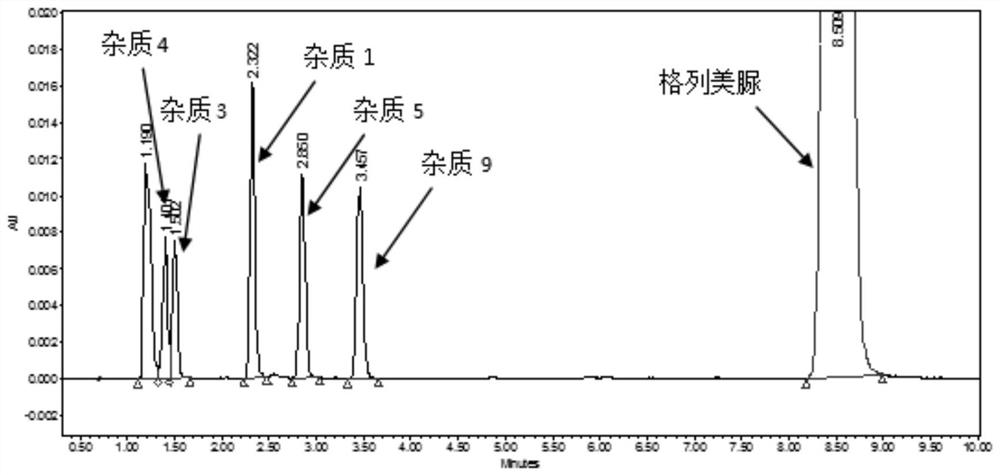
ActiveCN114839286AIncrease the number ofEasy to separateComponent separationAgainst vector-borne diseasesFluid phaseGlimepiridum
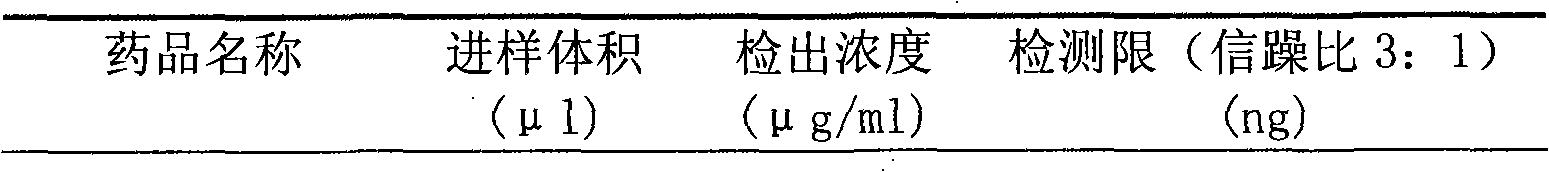
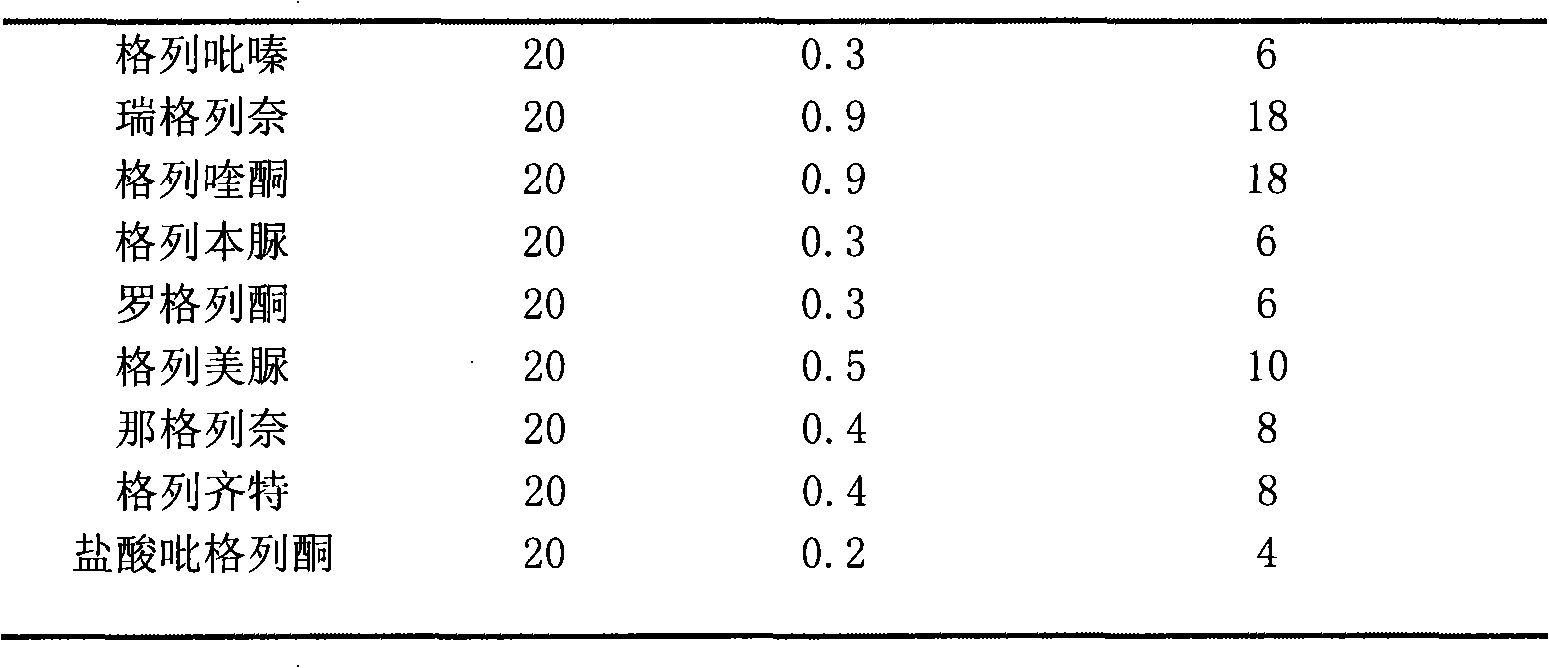
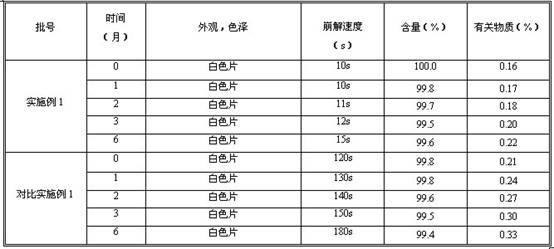
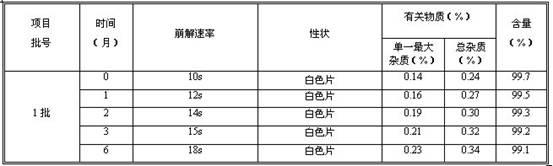
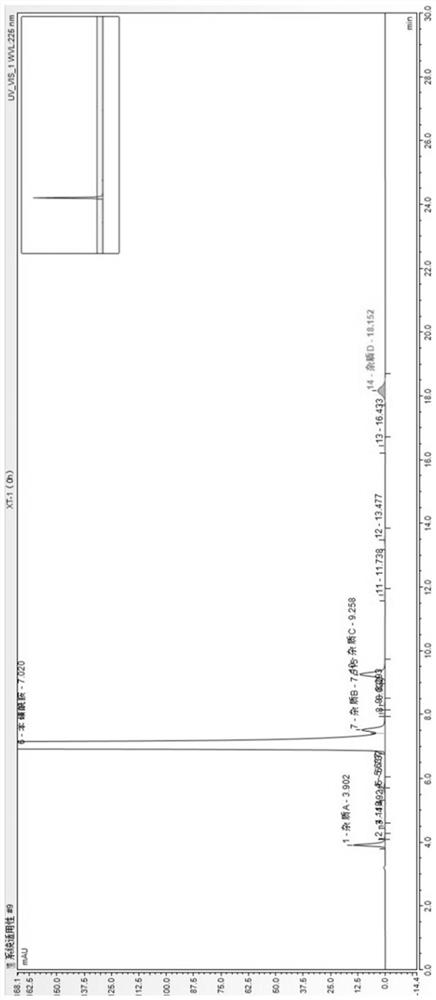
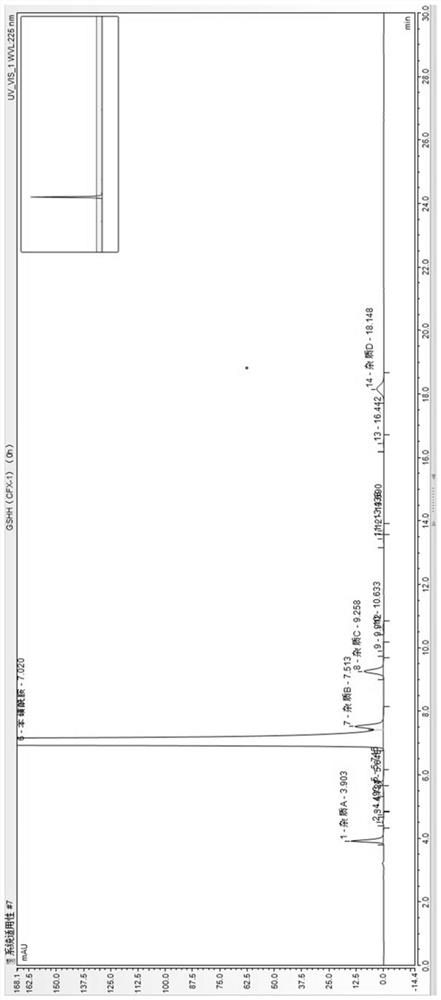
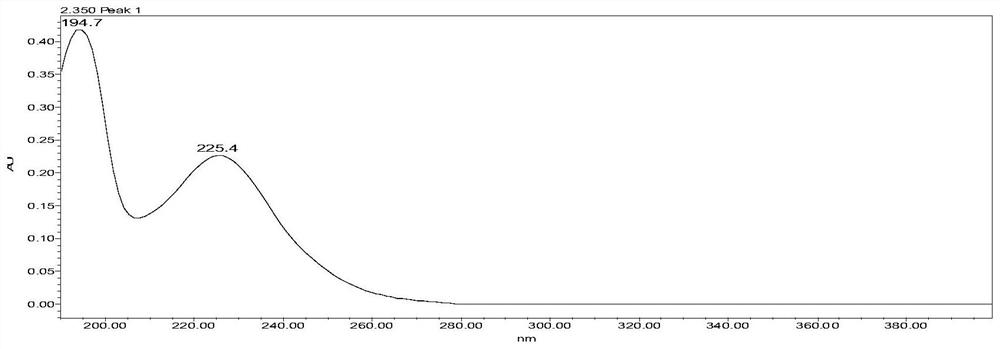
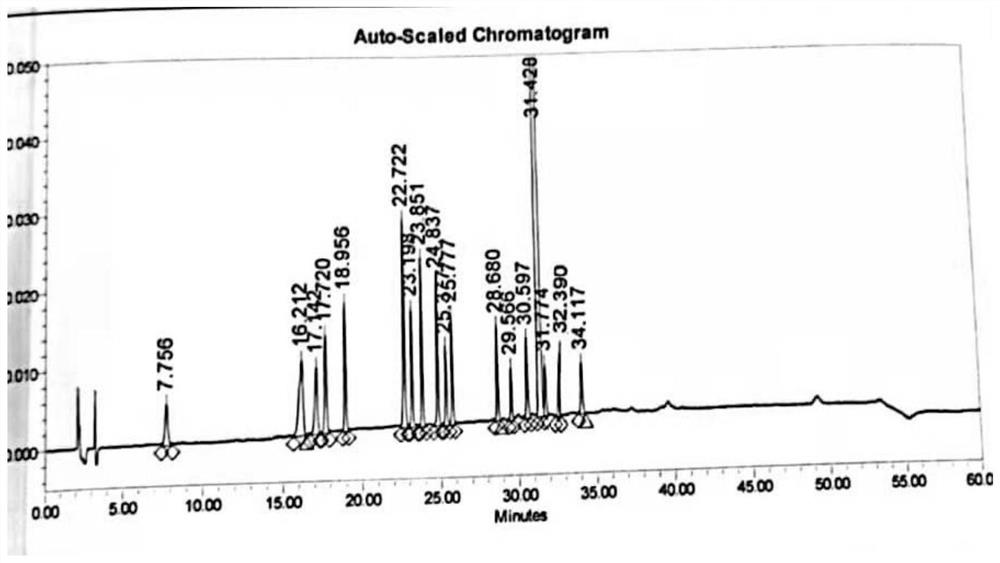
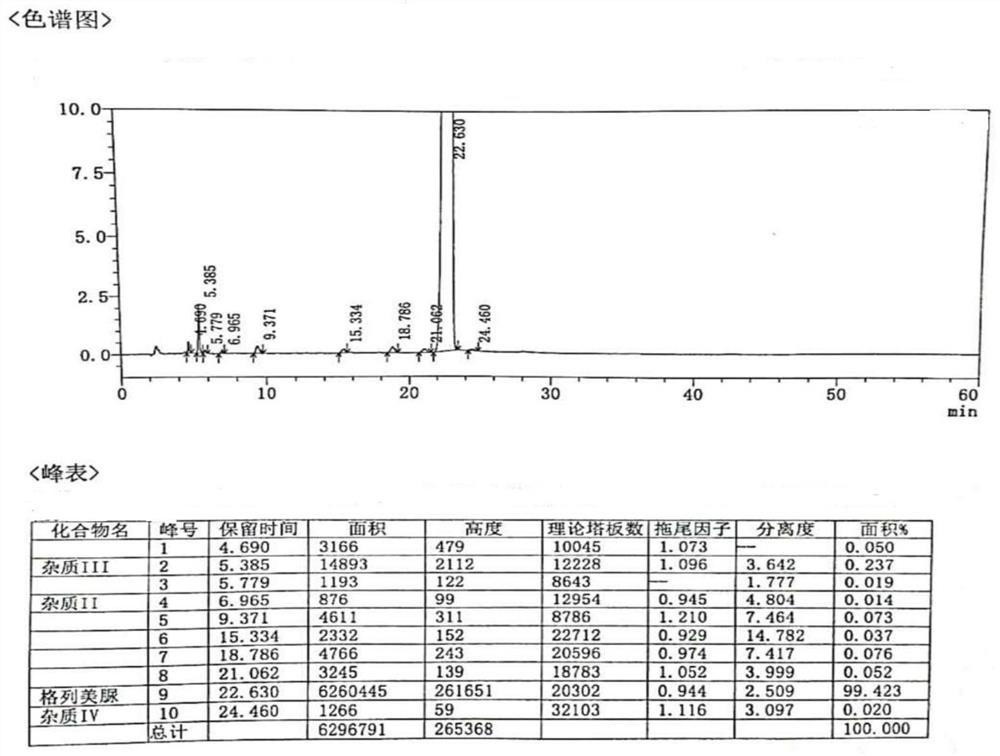
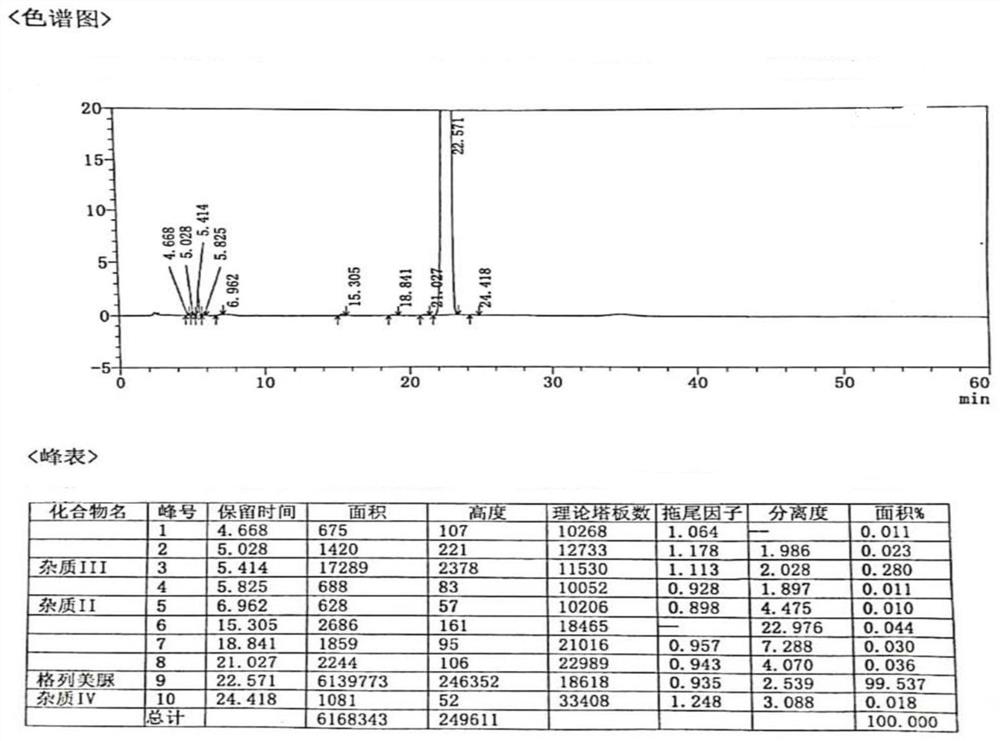
The invention provides a method for determining related substances in a glimepiride bulk drug. The method adopts a high performance liquid chromatography (HPLC) method and comprises the following steps: (1) preparing a test solution and a reference solution; (2) carrying out gradient elution by using a 0.1% sodium dihydrogen phosphate solution (the pH value is adjusted to 4.0 by using phosphoric acid) as a mobile phase A and acetonitrile as a mobile phase B; and (3) respectively injecting the test solution and the reference solution. According to the method, 17 impurities in the glimepiride raw material medicine can be effectively separated, and the method has high system applicability and can be used for controlling related substances in the glimepiride raw material medicine.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
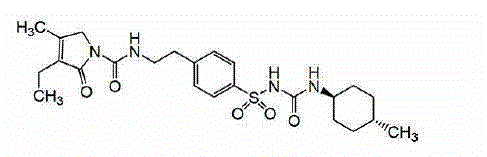
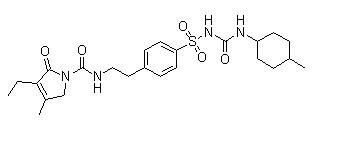
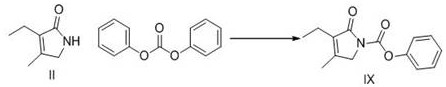
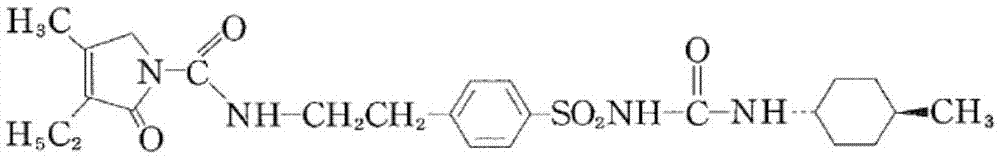
A kind of preparation method of glimepiride intermediate
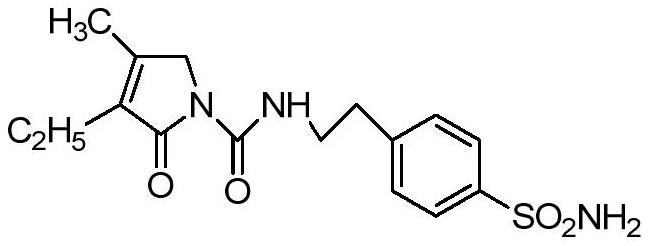
The invention relates to a preparation method of glimepiride intermediate compound I, which belongs to the technical field of preparation of raw materials. The preparation method of the present invention comprises the following steps: reacting compound II and diphenyl carbonate under the catalysis of a base (refer to Synlett, 28(18), 2495-2498; 2017) to prepare compound IX; then compound IX and 4‑(2‑Aminoethyl)benzenesulfonamide is fed at a ratio of 1:1, a weak acid is added, isopropanol is added, and the reaction is heated under reflux. The technical scheme of the present invention provides a method for preparing a high-purity glimepiride intermediate.
Owner:迪嘉药业集团股份有限公司
Preparation method for glimepiride tablet
InactiveCN111249242APrescription process optimizationChange solubilityMetabolism disorderSulfonylurea active ingredientsGlimepiridumPhysical chemistry
The invention relates to a preparation method for a glimepiride tablet. A formula technology of the glimepiride tablet is optimized, working procedures of granulation and drying are combined and simplified, glimepiride raw materials and lactose are added together as base materials, the glimepiride raw materials and the lactose are subjected to particle size regulation and control, proper mist spray pressure is adopted, and the dissolving property of raw materials in a fluidization and granulation process generates unexpected change, i.e., a dissolution rate can be guaranteed. Meanwhile, a similarity between an own product and a plurality of dissolution curves of a reference preparation can be improved, and an in vivo equivalence possibility with the reference preparation is improved.
Owner:CHONGQING CONQUER PHARML
Glimepiride tablet and preparation method thereof
ActiveCN106913545APromote dissolutionSimple preparation processMetabolism disorderSulfonylurea active ingredientsAcetic acidGlimepiride
The invention discloses a glimepiride tablet and a preparation method thereof. The preparation method comprises the following steps: dissolving glimepiride in a sodium hydroxide solution; adding mesoporous silica and carrying out uniform mixing under stirring; adding glacial acetic acid into a solution obtained in the previous step under stirring; allowing a compound of glimepiride and mesoporous silica to be precipitated and carrying out filtering and drying; then mixing the dried compound with a filler and a disintegrating agent and carrying out granulation and drying; and adding a lubricant into dry particles and then carrying out tabletting. Compared with the prior art, the glimepiride tablet prepared in the invention can be rapidly dissolved out in water, and the preparation method is simple in process and suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
A kind of method for preparing glimepiride crystal form I
ActiveCN110218171BDoes not affect the crystallization effectIncreased speed of crystallizationOrganic chemistry methodsAcetic acidAlcohol
The invention relates to a preparation method of glimepiride crystal form I, which belongs to the technical field of preparation of raw materials. The preparation method of the Limepiride crystal form I of the present invention comprises the following steps: dissolving the crude glimepiride in an aqueous alcohol solution, adding an appropriate amount of ammonia water, stirring to dissolve; filtering, adding acetic acid to the filtrate, and neutralizing the filtrate Ammonia in the crystalline form II, to obtain glimepiride crystal form II; to the crystal form II, add a ketone solvent 4 to 8 times its mass, stir at a temperature range of 10 to 35 °C for more than 10 hours, filter, and bake at 70 °C dry to obtain the glimepiride crystal form I product. The invention obtains a high-quality glimepiride crystal form I bulk drug.
Owner:迪嘉药业集团股份有限公司
Refining method of glimepiride bulk drug
InactiveCN112028807AImprove solubilityImprove purification efficiencyOrganic chemistryGlimepiridumMedicine
The invention provides a refining method of a glimepiride bulk drug, which comprises the following steps: (1) dissolving a glimepiride crude product in purified water, and filtering to obtain a filtrate, (2) acidifying the filtrate to generate slurry, filtering to obtain filter cake, and leaching the filter cake with purified water until the filter cake is neutral, and (3) adding the filter cake into a solvent, stirring, heating to reflux, keeping the reflux condition for 1-2 hours, filtering while the filter cake is hot, leaching with acetone, draining and drying to obtain the glimepiride fine medicine meeting the medicinal standard. The refining method of the glimepiride bulk drug provided by the invention is high in purification efficiency, simple and convenient in operation steps, mildin process conditions, low in production cost and more suitable for large-scale production.
Owner:CHONGQING CONQUER PHARML
Method for detecting glimepiride
PendingCN112345674AReduce distractionsShorten detection timeComponent separationGlimepiridumInternal standard
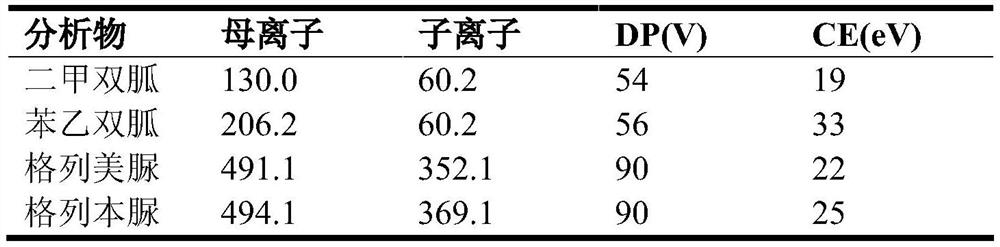
The invention provides a glimepiride detection method which comprises the following steps: preparing standard solutions with at least three concentrations of glimepiride and internal standard substances, and ensuring that the quantities of the internal standard substances in the standard solutions are the same; detecting each standard solution by using a liquid chromatograph-mass spectrometer under a detection condition to obtain a first detection result corresponding to the standard solution; simulating a standard curve equation of qualified glimepiride according to each first detection result, the concentration of glimepiride in the standard solution and the concentration of an internal standard substance; taking a first supernatant of a sample to be treated after centrifugation; addingthe internal standard substance into the first supernatant, uniformly mixing in a vortex manner, sequentially adding an extracting agent, and extracting the first supernatant to obtain a sample to bedetected; detecting the sample to be detected by utilizing the liquid chromatograph-mass spectrometer under the detection condition to obtain a second detection result of the sample to be detected; and based on the standard curve equation and the second detection result, obtaining the concentration of glimepiride in the sample to be detected. The scheme can shorten the sample detection time.
Owner:济南和合医学检验有限公司
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample tobe tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Rapid pretreatment method for simultaneously detecting glimepiride and metformin in blood plasma
ActiveCN111693636AGood magnetic responseSimplified processing stepsComponent separationOther chemical processesGlimepiridumBlood plasma
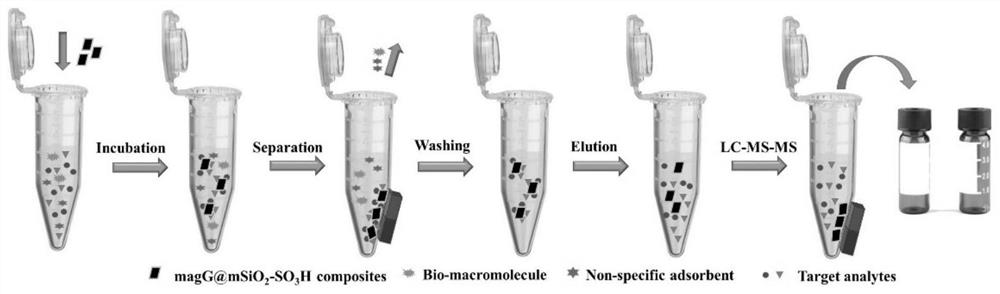
The invention belongs to the technical field of analysis, and relates to a rapid pretreatment method for detecting glimepiride and metformin in blood plasma, in particular to a rapid pretreatment method for enriching and detecting glimepiride and metformin in blood plasma based on a magnetic mesoporous graphene composite material. According to the method, the functionalized magnetic mesoporous graphene material is synthesized, and is used for the pretreatment process of the plasma sample and the method comprises the steps of extraction, cleaning, elution and detection, the sample treatment steps are obviously simplified, and the sample treatment time is shortened; the method can effectively eliminate interference caused by a matrix effect, is wide in linear range, good in recovery rate andhigh in sensitivity, can be used for simultaneously detecting the concentrations of glimepiride and metformin in blood plasma, and is further used for in-vivo pharmacokinetic analysis of glimepirideand metformin.
Owner:FUDAN UNIV
Self-nanoemulsifying 3D-printed tablet composition and method of use thereof
ActiveUS11253481B1Improve bioavailabilitySulfonylurea active ingredientsNanomedicineGlimepiridumComputer printing
Provided are a self-nanoemulsifying 3D printer ink composition and a method of using such composition to manufacture a 3D-printed tablet having compartmentalized active pharmaceutical ingredients. In particular, the 3D-printed tablet composition includes glimepiride and / or rosuvastatin in a curcuma oil based self-nanoemulsifying drug delivery system (SNEDDS).
Owner:KING ABDULAZIZ UNIV
Preparation method of glimepiride impurity
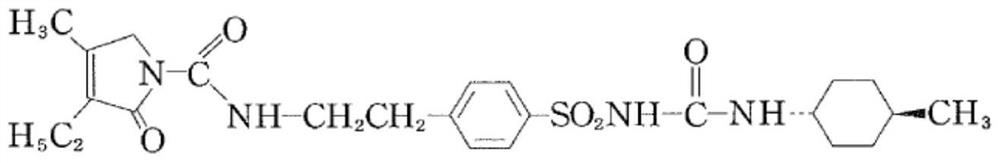
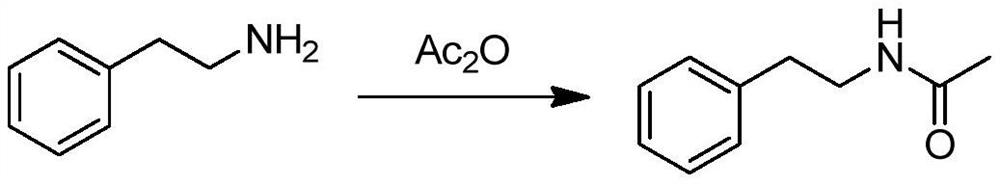
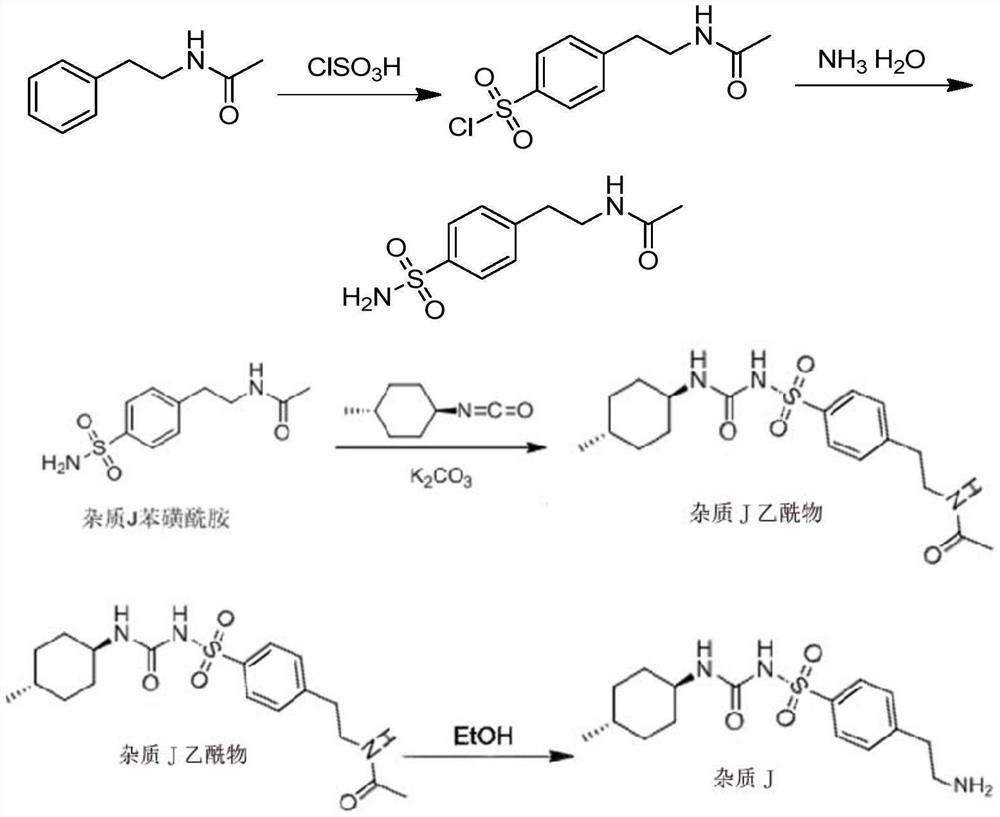
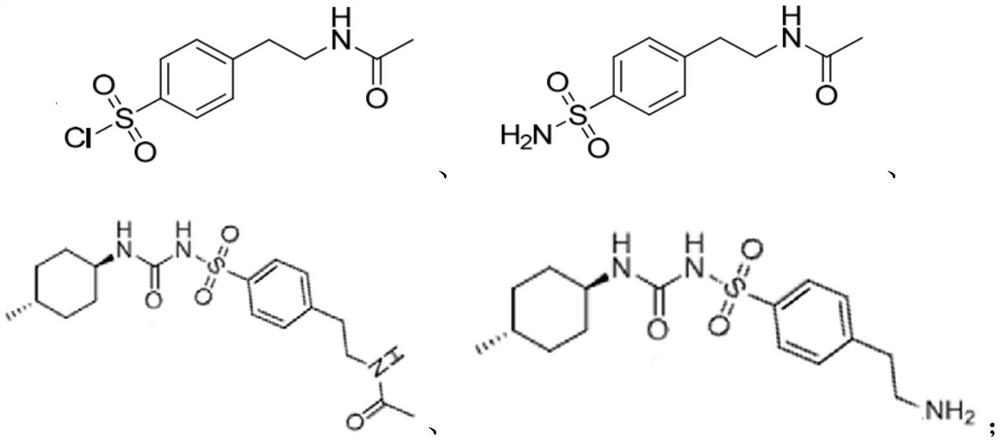
ActiveCN108299251BHigh purityRaw materials are easy to getOrganic chemistry methodsSulfonic acid amide preparationSulfonyl chlorideAcetic anhydride
Owner:SHANDONG XINHUA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com