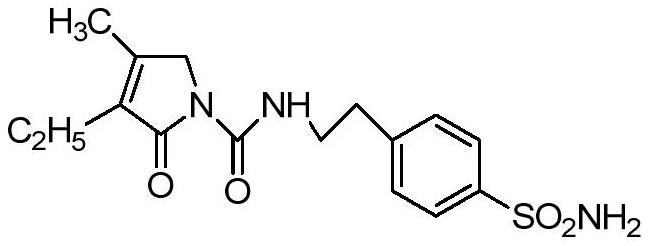
Analysis method for determining related substances in glimepiride intermediate by utilizing HPLC (High Performance Liquid Chromatography)
An analytical method and technology for related substances are applied in the field of chemical drug analytical method development to achieve the effects of convenient operation, high sensitivity and stability, and efficient elution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
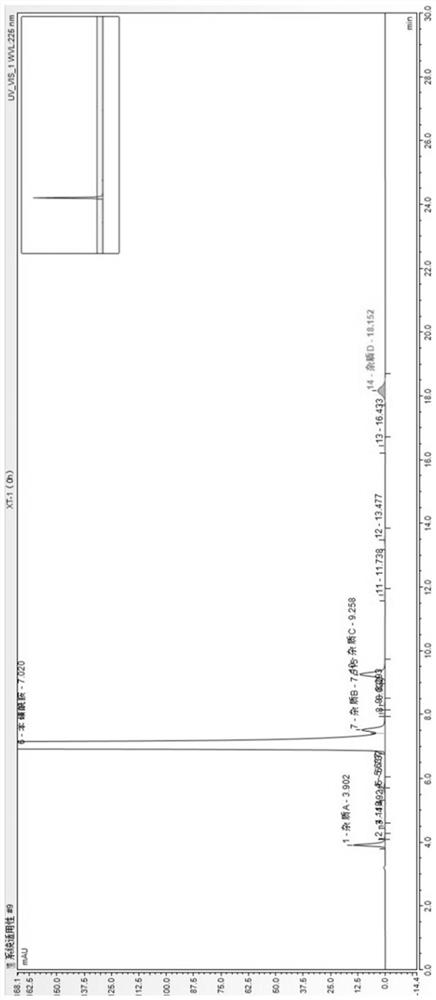
[0048] Comparison of different chromatographic columns in Example 1
[0049] HPLC conditions:
[0050] Performed on Agilent Poroshell 120PFP column (150*4.6mm, 2.7μm), Welch UltimateAQ-C18 column (250*4.6mm, 5μm), Welch Ultimate XB-C18 column (250*4.6mm, 5μm) In the test, the volume ratio of 0.01mol / L ammonium dihydrogen phosphate buffer solution with a pH of 3.5 adjusted by phosphoric acid and methanol was 50:50 as the mobile phase, the detection wavelength was 225nm, and the flow rate was 1.0ml / min or 0.5ml / min. The column temperature was 25°C, the injection volume was 20 μl, and isocratic elution was performed.
[0051] Sample preparation:
[0052] System suitability solution: Weigh appropriate amount of benzenesulfonamide reference substance and each impurity reference substance to prepare each 1ml containing about 0.1 mg of benzenesulfonamide reference substance, 0.5 μg of impurity A, 0.5 μg of impurity B, 0.5 μg of impurity C and impurities. D 0.5μg mixed solution.
...
Embodiment 2
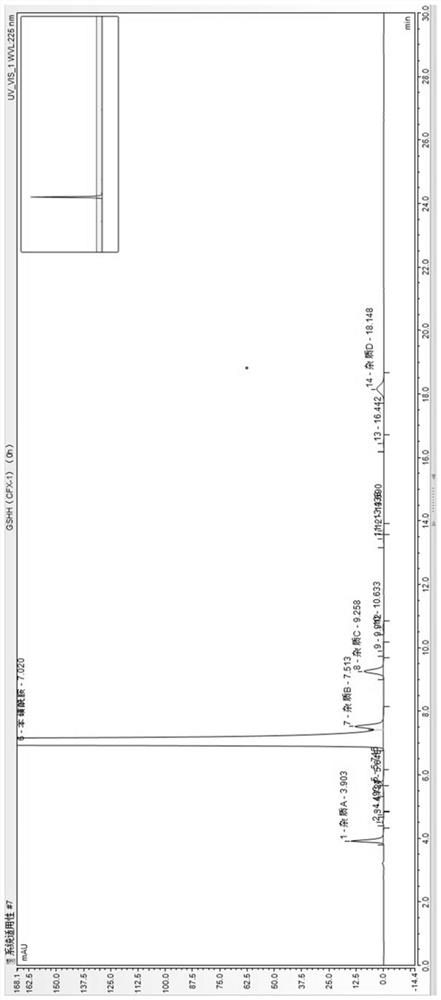
[0057] The contrast of embodiment 2 organic phase
[0058] HPLC conditions:
[0059] Methanol was used as the organic phase and acetonitrile was used as the organic phase. The chromatographic column was an Agilent Poroshell 120PFP column (150*4.6mm, 2.7μm), which was buffered with 0.01mol / L ammonium dihydrogen phosphate with a pH of 3.5 adjusted with phosphoric acid. The volume ratio of the solution and the organic phase was 50:50 as the mobile phase, the detection wavelength was 225 nm, the flow rate was 0.5 ml / min, the column temperature was 25 °C, the injection volume was 20 μl, and isocratic elution was performed.
[0060] The sample preparation is the same as in Example 1.
[0061] Test operation: Take 20 μl of system suitability solution and inject respectively, and record the chromatogram. The results are shown in Table 2.
[0062] Table 2 Summary table of organic phase screening results
[0063]
[0064] As can be seen from Table 2, either methanol or acetonitri...
Embodiment 3
[0065] Example 3 Comparison of mobile phase ratios
[0066] HPLC conditions:
[0067] The mobile phase ratios were tested respectively. The chromatographic column was an Agilent Poroshell 120PFP column (150*4.6mm, 2.7μm), and a mixture of 0.01mol / L ammonium dihydrogen phosphate buffer solution with a pH of 3.5 adjusted with phosphoric acid and acetonitrile was used. The solvent was the mobile phase, the detection wavelength was 225 nm, the flow rate was 0.5 ml / min, the column temperature was 25 °C, the injection volume was 20 μl, and isocratic elution was performed.
[0068] The sample preparation is the same as in Example 1.
[0069] Test operation: Take 20 μl of system suitability solution and inject respectively, and record the chromatogram. The results are shown in Table 3.
[0070] Table 3 Summary table of mobile phase ratio screening results
[0071]
[0072]
[0073] As can be seen from Table 2, when the volume ratio of ammonium dihydrogen phosphate buffer sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com