Preparation method for glimepiride tablet
A technology of Glimex and urea tablets, which is applied in the field of medicine, can solve the problems of poor similarity of dissolution curves of reference preparations, high requirements for control of excipients, and poor similarity of dissolution curves, etc., to optimize the prescription process, improve similarity, Guaranteed dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
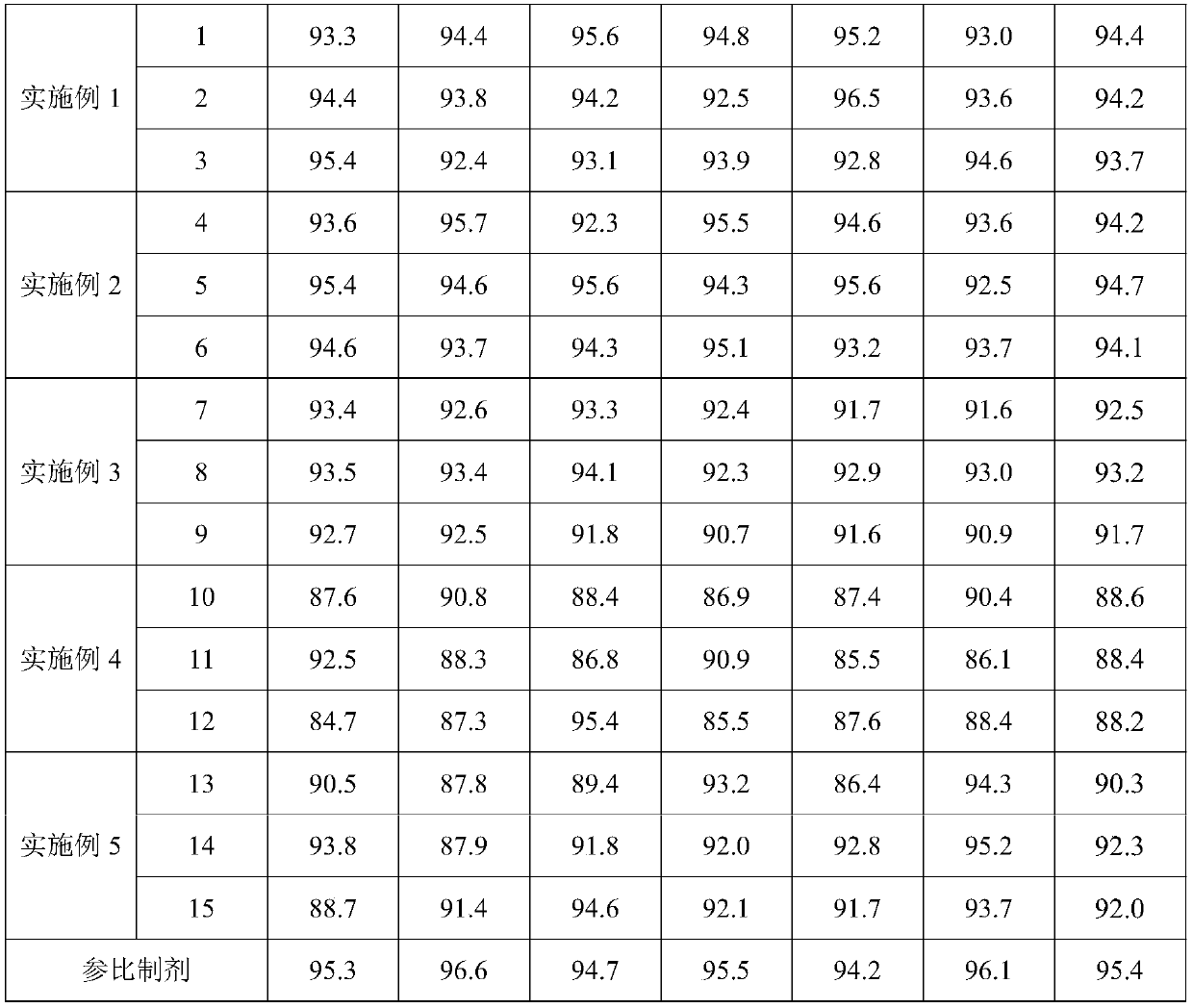
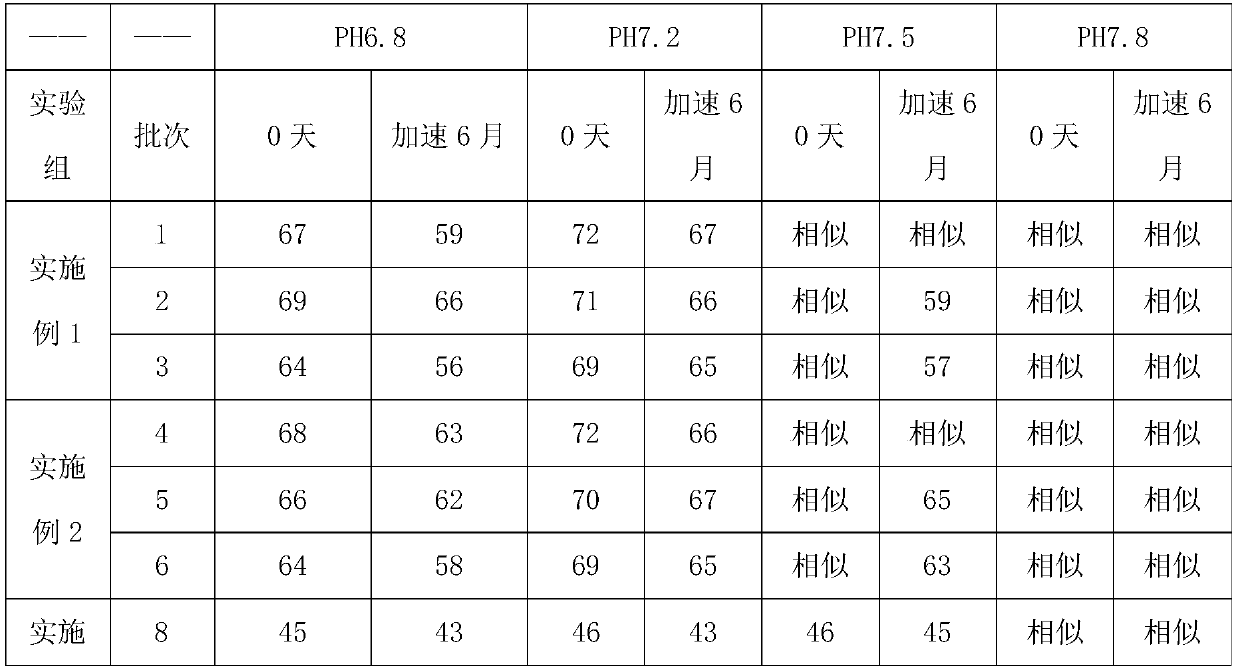
Examples
Embodiment 1
[0066] A preparation method of glimepiride tablet, comprising the steps of:
[0067] (1) 30g of glimepiride raw material is pulverized with a universal pulverizer to obtain ordinary powder with a particle size D90 of about 150 μm;
[0068] (2) 15g povidone is configured into an aqueous solution of 15% concentration;
[0069] (3) Pass 30g of glimepiride raw material together with 1.8kg of lactose and 300g of sodium starch glycolate through a 100-mesh sieve and add to the fluidized bed;
[0070] (4) set fluidized bed inlet air temperature, fan frequency, spray pressure and liquid spray speed, spray povidone aqueous solution into fluidized bed for granulation, and dry;
[0071] The specific setting parameters are as follows: air intake volume 20HZ, air intake temperature 60°C, spray pressure 0.2Mpa, liquid spray speed 250r / min,
[0072] (5) After the dried granules are crushed and granulated with a 20-mesh granulator, 3 g of microcrystalline cellulose and 15 g of magnesium stea...
Embodiment 2
[0074] A preparation method of glimepiride tablets, comprising the following steps: (1) 25 g of glimepiride raw materials are pulverized by a universal pulverizer to obtain micropowder with a particle diameter D90 of less than 10 μm;
[0075] (2) 40g povidone is configured into 20% concentration of ethanol aqueous solution (2:3);
[0076] (3) 25g glimepiride, 1.8kg lactose, and 400g carboxymethyl starch sodium are passed through a 100-mesh sieve and added to the fluidized bed;
[0077] (4) Appropriate fluidized bed air inlet temperature, fan frequency, spray pressure and spray speed are set, and the povidone aqueous solution is sprayed into it to granulate and dry;
[0078] The specific setting parameters are as follows: air intake volume 20HZ, air intake temperature 50°C, spray pressure 0.3Mpa, liquid spray speed 300r / min.
[0079] (5) After the dried granules are crushed and granulated with a 20-mesh granulator, 20 g of microcrystalline cellulose and 40 g of magnesium stear...
Embodiment 3
[0081] Preparation method of prior art glimepiride tablets (our patent number is CN105769787A)
[0082] Micropowder the glimepiride raw material to below 10 μm, weigh 2kg of the glimepiride raw material, 55kg of lactose, 5.2kg of sodium carboxymethyl starch I (internal addition), 0.4kg of povidone, and 0.25kg of microcrystalline cellulose, Carboxymethyl starch sodium II (external addition) 1.9kg, magnesium stearate 0.325kg, glimepiride micropowder, lactose, carboxymethyl starch sodium I (internal addition) were added to the wet granulator and mixed for 20-30 minutes to obtain Glide meurea mixture;
[0083] (2) Add povidone into 9.6kg water and dissolve, and be mixed with 4% povidone aqueous solution (W / W);
[0084] (3) Add 4% povidone aqueous solution to make soft material in the wet granulator, add 14 mesh screens to granulate with a swing granulator, and dry the granules with a fluidized bed;
[0085] (4) Grind the dried granules with a 20 mesh, add microcrystalline cellul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com