Preparation method of glimepiride tablets
A technology of glimeide and urea tablets, applied in the field of medicine, can solve the problems of poor similarity of the dissolution curve of the reference preparation, poor similarity of the dissolution curve, complicated preparation process, etc. Effects of chemical process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
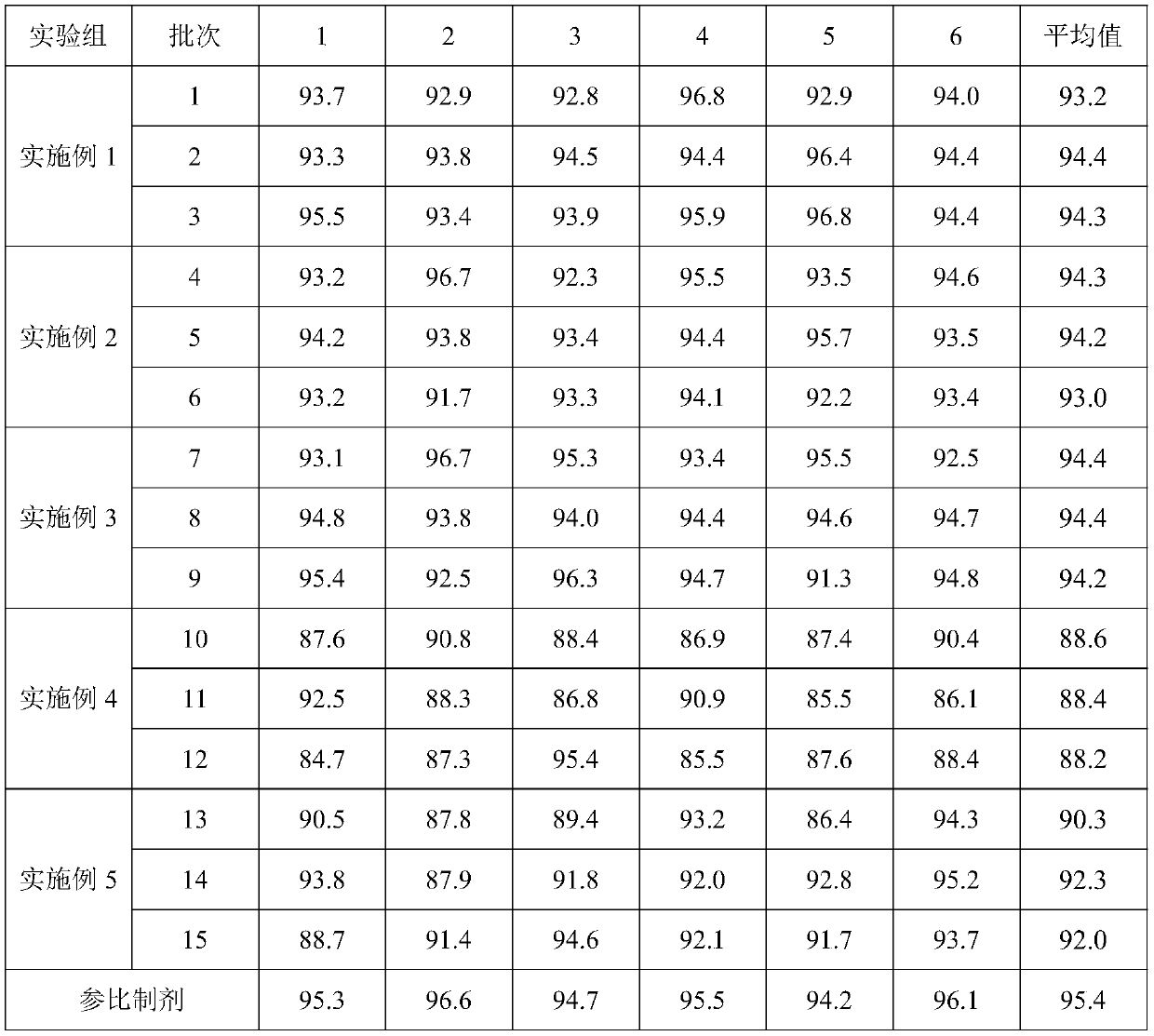
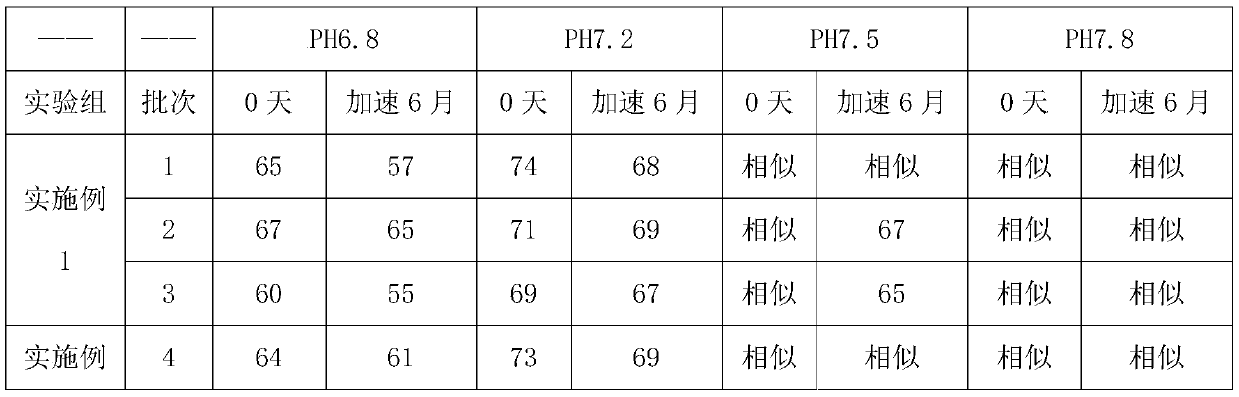
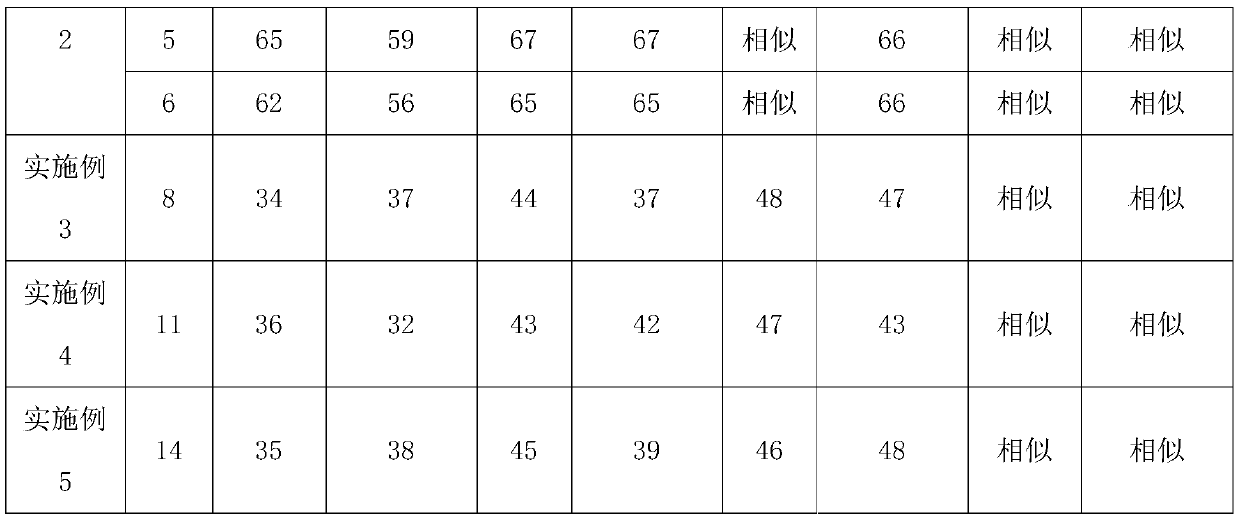
Embodiment 1
[0058] A kind of preparation method of glimepiride tablet comprises the steps:
[0059] (1) The glimepiride raw material selects fine powder with a particle diameter D90 obtained by jet milling that is less than 5 μm;
[0060] Add 2kg of glimepiride, 65kg of lactose, 0.8kg of povidone, and 15kg of carboxymethyl starch sodium into a wet granulator and mix for 20 minutes, then add 10kg of purified water to make soft materials and shear and mix for 10 minutes, and then use the YK160A rocking granulator grain;
[0061] (2) Dry the wet granules with a boiling dryer at 80°C for 16 minutes, with a moisture content of 1.3%, and then pass through a crushing and sizing machine with a 20-mesh granulation, add 0.8 kg of microcrystalline cellulose, 0.5 kg of magnesium stearate and mix for 5 minutes, mix Speed 24rpm;
[0062] (3) When pressing the tablet, control the pressure to 6-9KN, and the hardness of the plain tablet is about 6kgf.
Embodiment 2
[0064] A preparation method of glimepiride tablet, comprising the steps of:
[0065] (1) The particle size D90 obtained by jet milling of raw materials is 5-10 μm;
[0066] Add 1.2kg of glimepiride, 90kg of lactose, 2kg of povidone, and 20kg of carboxymethyl starch sodium into a wet granulator and mix for 20 minutes, then add 10kg of purified water to make soft materials and shear and mix for 30 minutes, and then use the YK160A rocking granulator grain;
[0067] (2) Dry the wet granules with a boiling dryer at 80°C for 20 minutes, with a moisture content of 0.8%, and then pass through a 20-mesh crushing and sizing machine, add 1 kg of microcrystalline cellulose and 2 kg of magnesium stearate and mix for 5 minutes at a mixing speed of 24 rpm ;
[0068] (3) ZP35B tablet press machine, the control pressure is 3-6KN when pressing the tablet, and the hardness of the plain tablet is about 3kgf. Embodiment 3 (comparative example)
Embodiment 3
[0068] (3) ZP35B tablet press machine, the control pressure is 3-6KN when pressing the tablet, and the hardness of the plain tablet is about 3kgf. Embodiment 3 (comparative example)
[0069] A preparation method of glimepiride tablet, comprising the steps of:
[0070] (1) The particle size D90 obtained by jet milling of raw materials is a micropowder below 2 μm;
[0071] Add 2kg of glimepiride, 90kg of lactose, 0.5kg of povidone, and 5kg of carboxymethyl starch sodium into a wet granulator and mix for 20 minutes, then add 10kg of purified water to make soft materials and shear and mix for 2 minutes, and then use the YK160A rocking granulator grain;
[0072] (2) Dry the wet granules with a boiling dryer at 80°C for 16 minutes, with a moisture content of 0.9%, and then pass through a crushing and sizing machine with a 20-mesh granulation, add 0.1 kg of microcrystalline cellulose and 0.5 kg of magnesium stearate and mix for 5 minutes, and mix Speed 24rpm;
[0073] (3) When ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com