Preparation method of telmisartan tablets
A technology of telmisartan and plain tablets, which is applied in the field of preparation of telmisartan tablets, can solve problems such as poor similarity, and achieve the effects of saving manpower and material resources, improving similarity, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A preparation method of telmisartan tablet, comprising the following steps:
[0027] S1, take telmisartan, sodium hydroxide and be mixed with aqueous solution or ethanol aqueous solution;
[0028] S2. Adjust the parameters of the fluidized bed, set the air inlet temperature to 80°C for spray drying and granulation, spray the mixed solution prepared in step S1, continue drying after spraying, control the moisture content to not be higher than 3.5%, and obtain dry granules, and the drying is completed After whole grain;
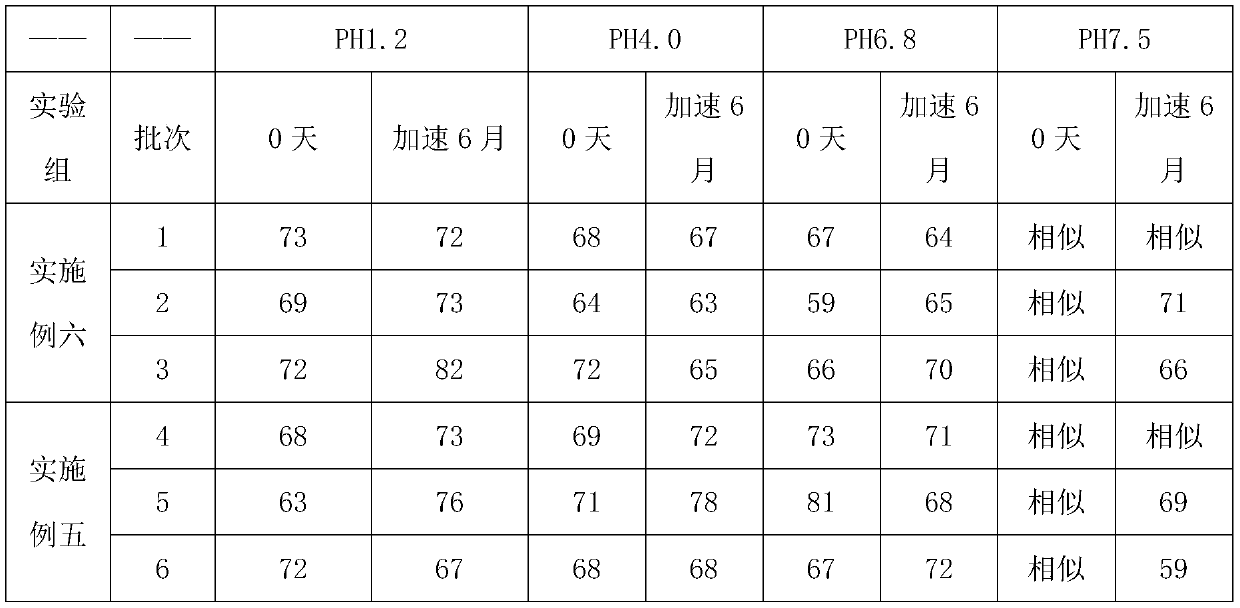
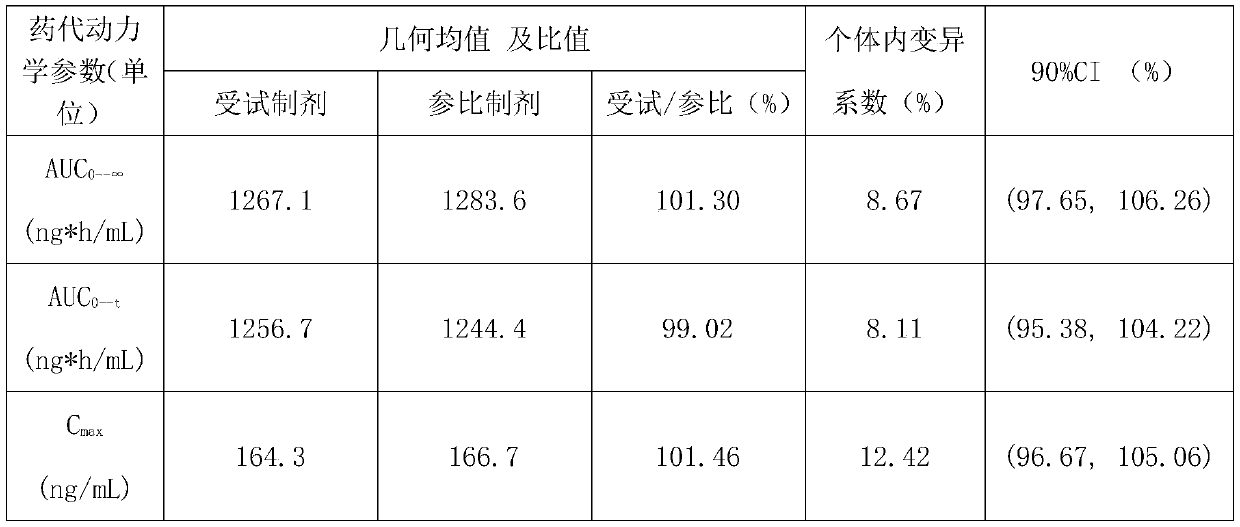
[0029] S3. Add the weighed mannitol, meglumine, and povidone to the sized granules, mix for 15 minutes, mix evenly with the weighed magnesium stearate and sodium stearyl fumarate, and then press into tablets to obtain vegetarian tablets. The hardness of the plain tablet is controlled at 9-11kgf;
[0030] S4. Film-coat the plain tablet.
[0031] Wherein, the weight ratio of telmisartan, mannitol, meglumine, povidone, sodium hydroxide, magnesium stearat...
Embodiment 1
[0035] A preparation method of telmisartan tablet, comprising the following steps:
[0036] S1, take by weighing telmisartan 50g, sodium hydroxide 4g and join in 200ml water to prepare an aqueous solution, set aside;
[0037] S2. Adjust the parameters of the fluidized bed, set the air inlet temperature to 100°C for spray drying and granulation, spray the aqueous solution prepared in step S1, continue drying after the spraying is completed, and control the moisture content to be no higher than 3.5%, to obtain dry granules. After the drying is completed Whole grain;
[0038] S3. Add 700g of mannitol, 30g of meglumine, and 30g of povidone to the granulated granules, mix them for 15min, mix them with 25g of magnesium stearate and 5g of sodium stearyl fumarate, and press Tablets are made of plain tablets, and the hardness of the plain tablets is controlled at 7-11kgf;
[0039] S4. Film-coat the plain tablet.
Embodiment 2
[0041] A preparation method of telmisartan tablet, comprising the following steps:
[0042] S1. Prepare 170ml of ethanol solution according to the ratio of ethanol and water at 2:1, weigh 50g of telmisartan and 4g of sodium hydroxide and add them to the prepared ethanol solution to prepare an aqueous ethanol solution for subsequent use;
[0043] S2. Adjust the parameters of the fluidized bed, set the air inlet temperature to 80°C for spray drying and granulation, spray into the ethanol aqueous solution prepared in step S1, continue drying after spraying, control the moisture content to not be higher than 3.5%, and obtain dry granules, and the drying is completed After whole grain;
[0044] S3. Add 75g of mannitol, 20.5g of meglumine, and 20.5g of povidone to the granulated granules, mix for 15 minutes, and mix with 2g of magnesium stearate and 6.25g of sodium stearyl fumarate After uniform compression, plain tablets are obtained, and the hardness of the plain tablets is contr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com