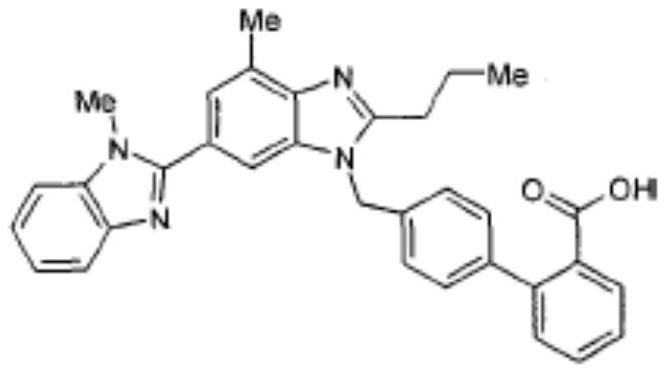
A kind of telmisartan tablet and preparation method thereof
A technology for telmisartan and tableting, applied in the field of telmisartan tablets and preparation thereof, can solve the problems of labor and material resources, viscosity of solution, harsh conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of preparation method of telmisartan tablet comprises the steps:
[0037] 1. Weigh 40g telmisartan, 3.3g sodium hydroxide, 12g meglumine, 12g povidone, 160g mannitol, 2.4g magnesium stearate, 3.6g sodium stearyl fumarate;
[0038] 2. The above-mentioned amount of telmisartan, sodium hydroxide, meglumine, and povidone are prepared into ethanol aqueous solution;
[0039] 3. Add 1 / 3 of the 20-mesh mannitol into the fluidized bed, the air inlet temperature is 60°C, and the spraying speed is 6r / min, so that it is in a fluidized state; the prepared 1 / 3 Spray 1 / 3 of the mixed solution into the fluidized bed and spray 1 / 3 of the mixed solution into the fluidized bed after the spray granulation is completed. After the granulation is completed, adjust the air inlet temperature to 100°C and the spray speed to 10r / min, add the remaining 1 / 3 of mannitol into the fluidized bed and spray the remaining 1 / 3 of the mixed solution, and dry to control the moisture to not exceed 2.5...
Embodiment 2
[0044] A kind of preparation method of telmisartan tablet comprises the steps:
[0045] 1. Weigh 80g telmisartan, 7.0g sodium hydroxide, 30g meglumine, 40g povidone, 300g mannitol, 3.6g magnesium stearate, 7.2g sodium stearyl fumarate;
[0046] 2. Prepare telmisartan, sodium hydroxide, meglumine, and povidone into ethanol aqueous solution;
[0047] 3. First add 1 / 3 of the mannitol sieved with 40 mesh into the fluidized bed, the inlet air temperature is 60°C, and the spraying speed is 6r / min, so that it is in a fluidized state; the prepared 1 / 3 Spray 1 / 3 of the mixed solution into the fluidized bed and spray 1 / 3 of the mixed solution into the fluidized bed after the spray granulation is completed. After the granulation is completed, adjust the air inlet temperature to 90°C, spray the slurry at a speed of 10r / min, add the remaining 1 / 3 of mannitol into the fluidized bed and spray the remaining 1 / 3 of the mixed solution, and dry to control the moisture to not exceed 2.5%. ;
...
Embodiment 3
[0051] Example 3 Preparation method I (control group 1) of prior art telmisartan tablets:
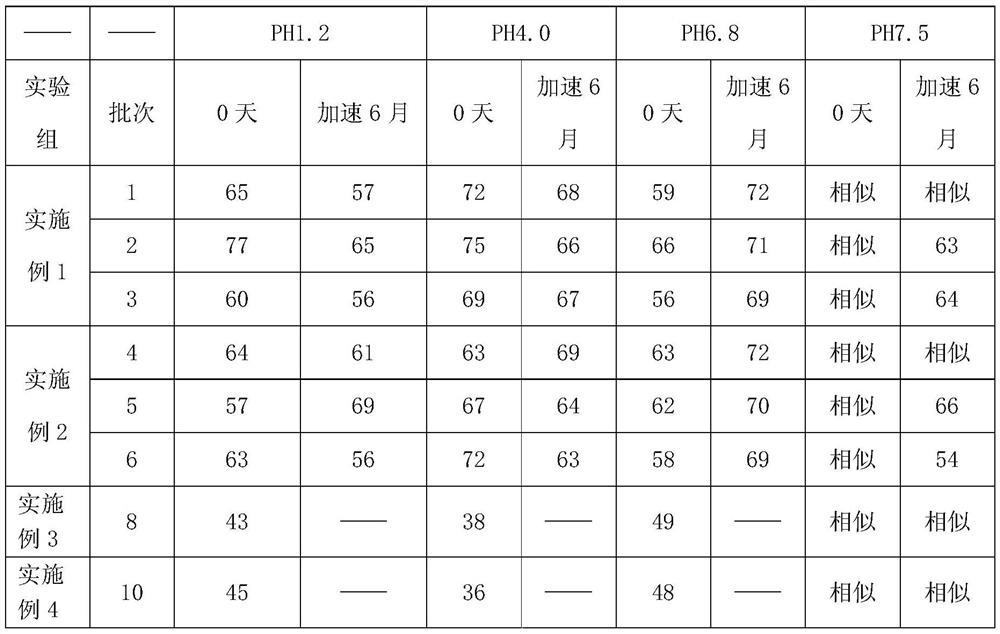
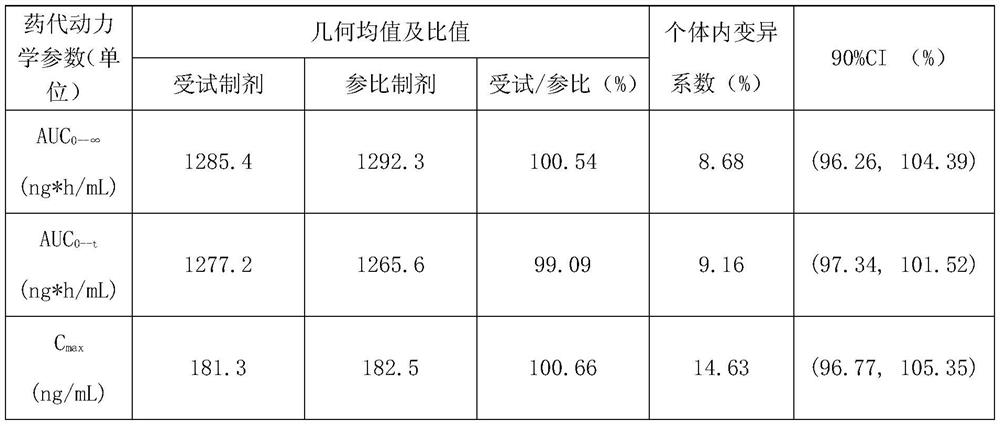
[0052] The sodium hydroxide 3.36g that weighed recipe quantity (in 1000 pieces) joins 150mL purified water, after stirring and dissolving, add meglumine 12g, after stirring and dissolving completely, add telmisartan raw material 40g, after stirring and dissolving completely, Finally, 12g of povidone was added, stirred and dissolved, and ultrasonically degassed to obtain a slurry. Weighed 122.24g of mannitol as a substrate and added it to a fluidized bed for granulation, plus 31.92g of mannitol, 12g of hydroxypropyl cellulose, and stearin Magnesium acid 1.68g, talcum powder 4.8g are mixed and compressed into tablets to obtain Telmisartan tablets. Among them, the parameters of the fluidized bed are: the spraying speed is 10r / min, the air inlet temperature is 50°C, the fan frequency is 30Hz, and the material temperature is controlled at about 40°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com