Glimepiride tablet and preparation method thereof
A technology of Glimepiride and Glimepiride, which is applied to medical formulations, sulfonylurea active ingredients, and medical preparations of non-active ingredients, etc., can solve problems such as long time required, difficult release, strong viscosity, etc., and achieve reduction Dosage, stable results, and the effect of improving hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
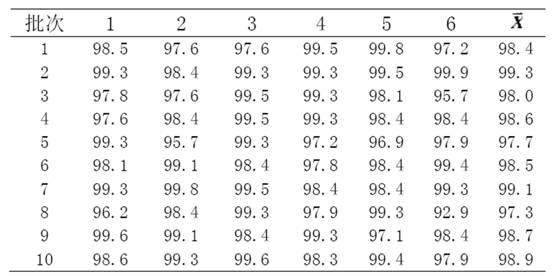
[0018] Example 1 Preparation Method I of Glimepiride Tablets
[0019] Weigh 20g of glimepiride raw material, 360g of lactose, 100g of sodium starch glycolate, 200g of microcrystalline cellulose, 20g of microcrystalline silica gel, and 5g of magnesium stearate; prepare the glimepiride raw material by means of inert gas flow pulverization become particle size less than 2 mu m of glimepiride powder;
[0020] The glimepiride powder was mixed and ground for 90 minutes by adding lactose in increments, then mixed with microcrystalline cellulose and ground again for 30 minutes, and the grinding temperature was lower than 30°C to obtain a glimepiride-lactose mixture;
[0021] In the glimepiride-lactose mixture, carboxymethyl starch sodium and micropowder silica gel were put into the wet granulator and mixed for more than 3 minutes, and the aqueous solution containing 0.5% hypromellose by mass ratio was added to the wet granulator in a ratio of 1:20 as a binder. granulator granulati...
Embodiment 2
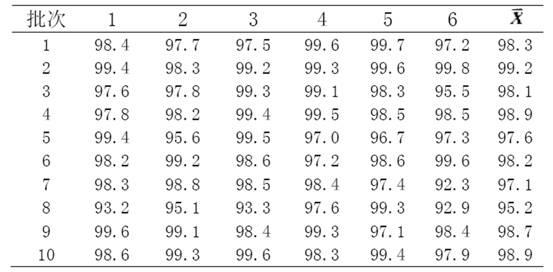
[0023] The preparation method II of embodiment 2 glimepiride sheet
[0024] Weigh 20g of glimepiride raw material, 360g of lactose, 100g of sodium starch glycolate, 200g of microcrystalline cellulose, 20g of microcrystalline silica gel, and 5g of magnesium stearate; prepare the glimepiride raw material by means of inert gas flow pulverization become particle size less than 2 mu m of glimepiride powder;
[0025] Glimepiride powder was mixed and ground for more than 60 minutes by adding lactose in increments, mixed with microcrystalline cellulose and ground again for more than 20 minutes, mixed with carboxymethyl starch sodium and micropowder silica gel and ground for more than 3 minutes, and the grinding temperature was lower than 30°C to obtain Glycine meurea mixture;
[0026] Add povidone dry powder into the glimepiride mixture and fully stir, mix and add magnesium stearate, control the hardness of the tablet press at 4 kg, and press the tablets to obtain glimepiride tabl...
Embodiment 3
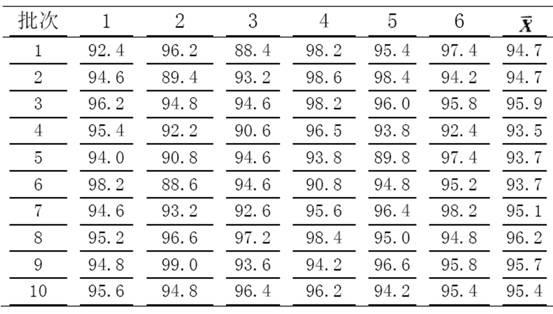
[0027] Example 3 Preparation method I of prior art glimepiride tablets
[0028] Weigh 20g of glimepiride raw material, 360g of lactose, 100g of sodium starch glycolate, 200g of microcrystalline cellulose, 20g of microcrystalline silica gel, and 3.5g of magnesium stearate; grind and mix the glimepiride raw material with the prescribed amount of lactose After about 2 hours, the mixture was ground and mixed with microcrystalline cellulose for 30 minutes, and then mixed with the prescribed amount of sodium carboxymethyl starch and micropowder silica gel on a wet granulator for 8 minutes. Dissolve an appropriate amount of Tween 80 in 95% ethanol, then mix it with 1% hypromellose aqueous solution at a ratio of 1:20 as a binder, add it to the mixture of the wet granulator for granulation, dry it and then mix it with the prescription amount Magnesium stearate was blended and tabletted to obtain glimepiride tablets;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com