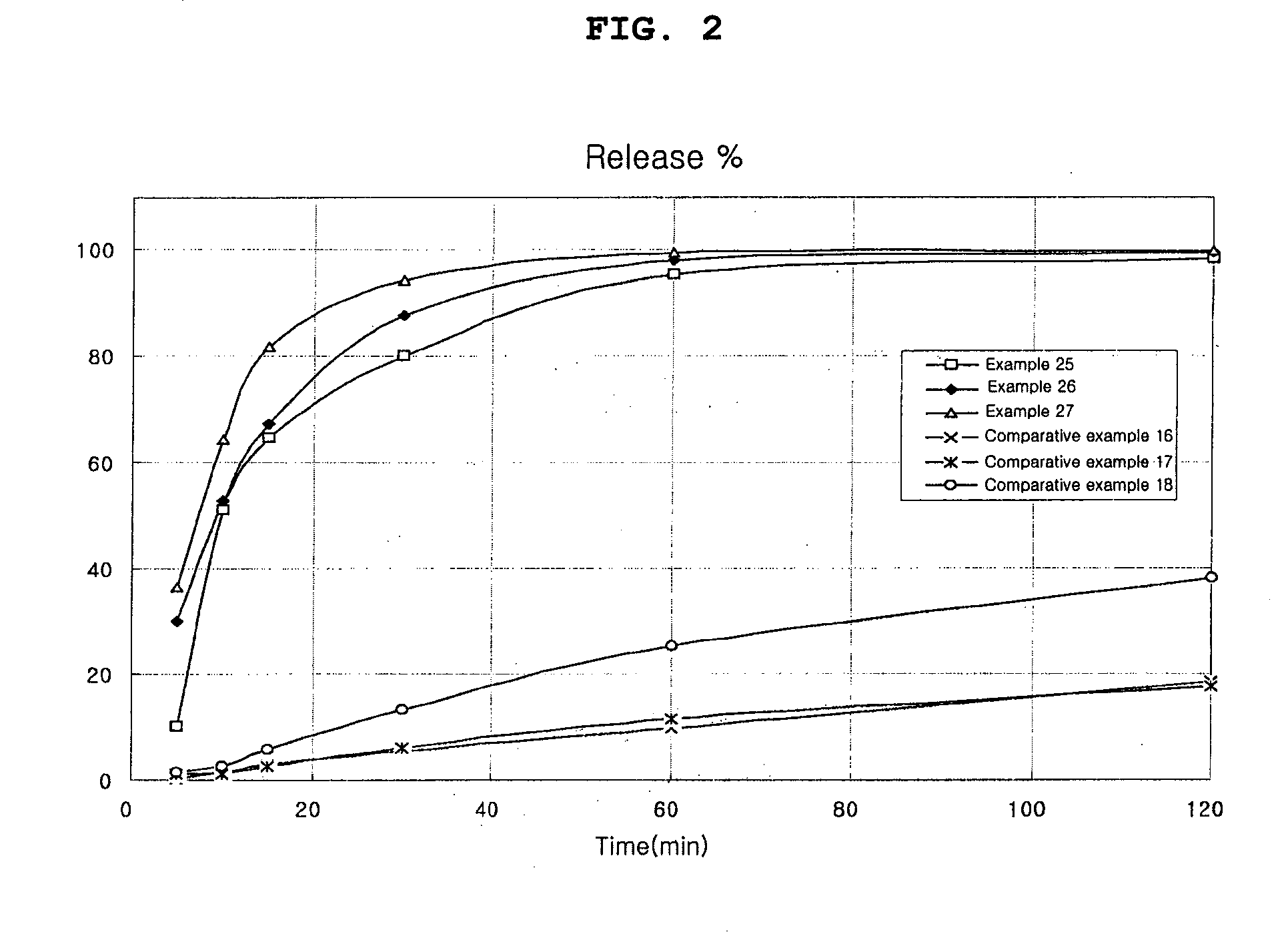
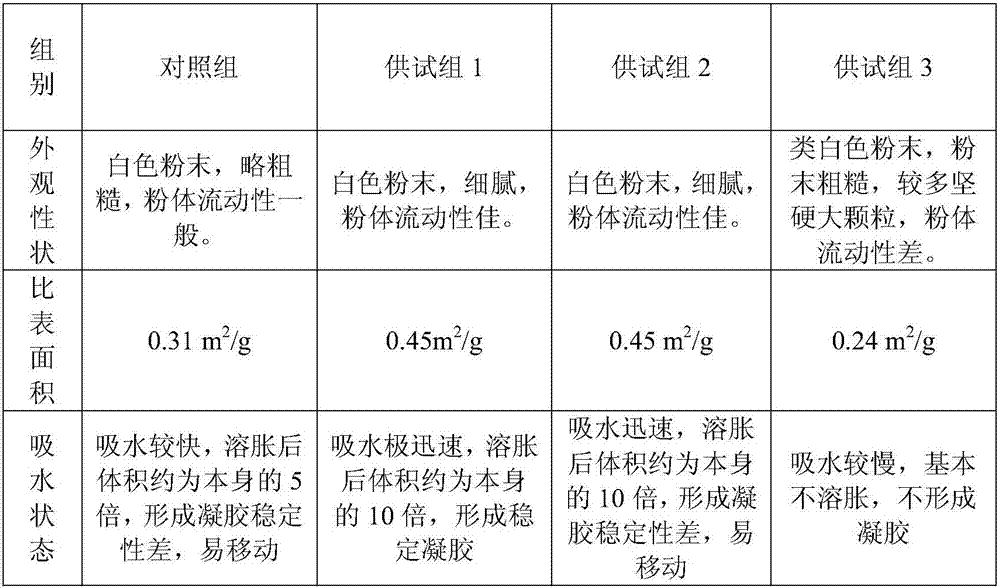
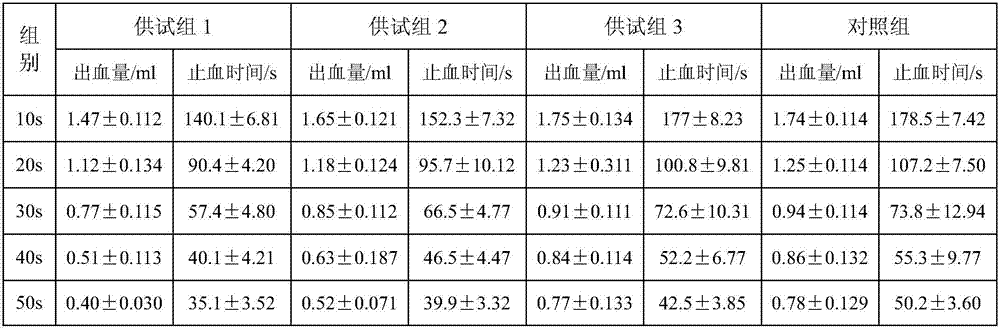
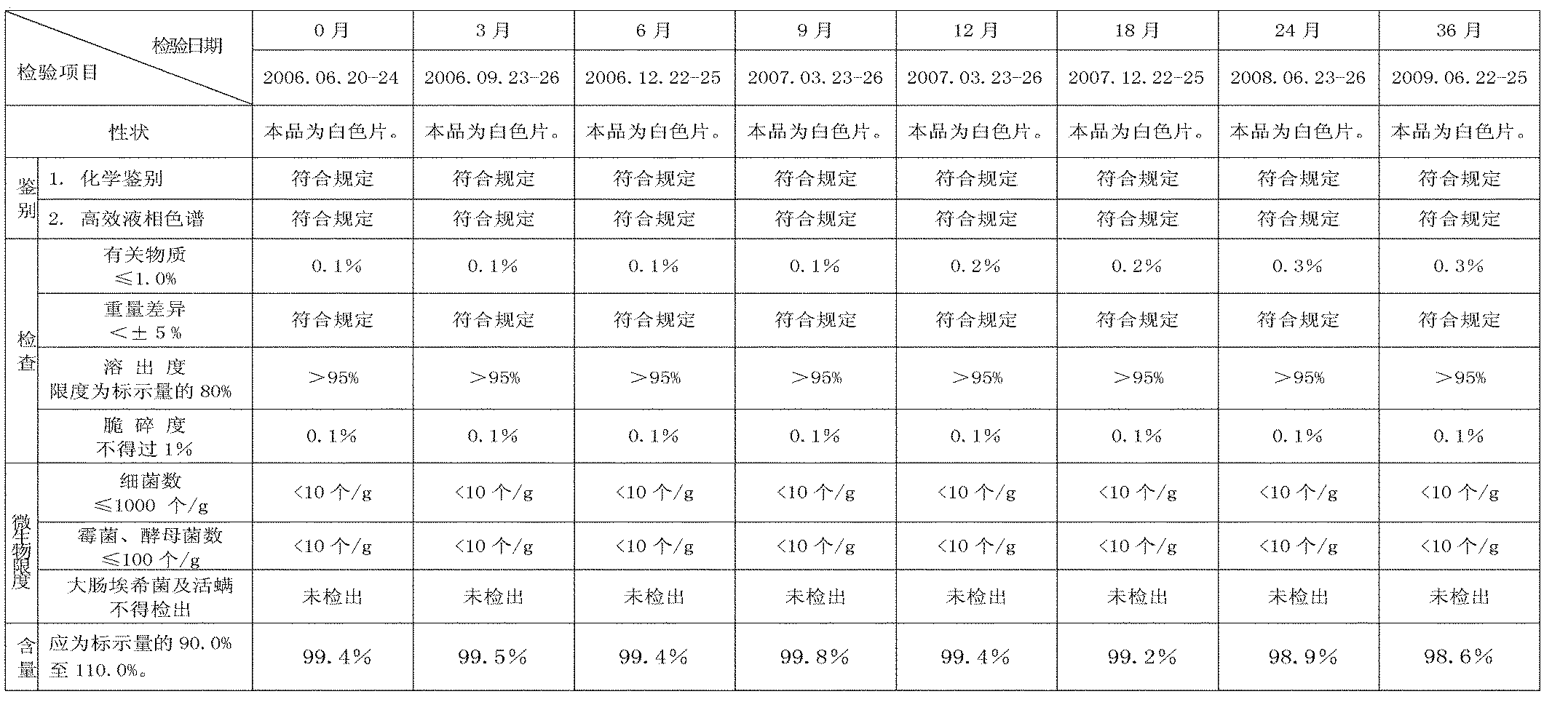
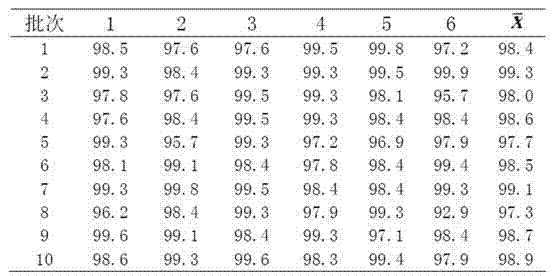
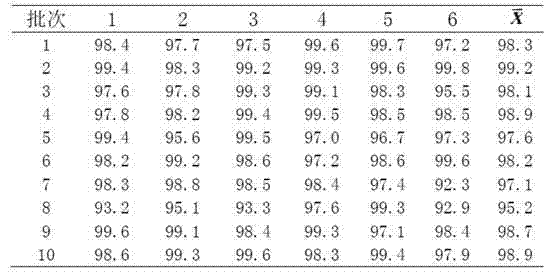
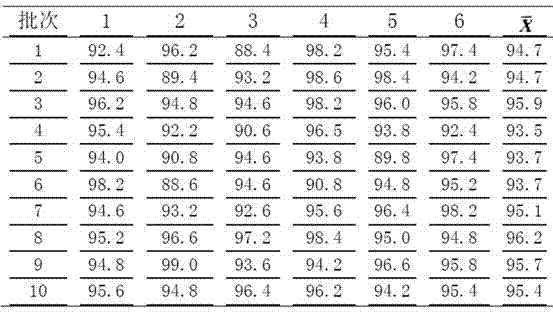
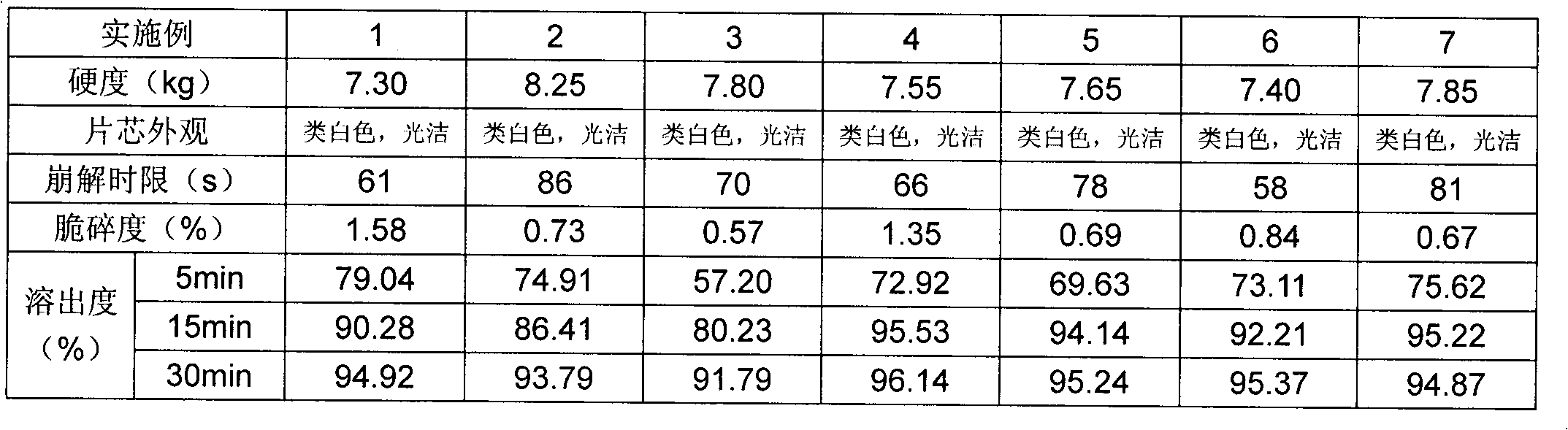
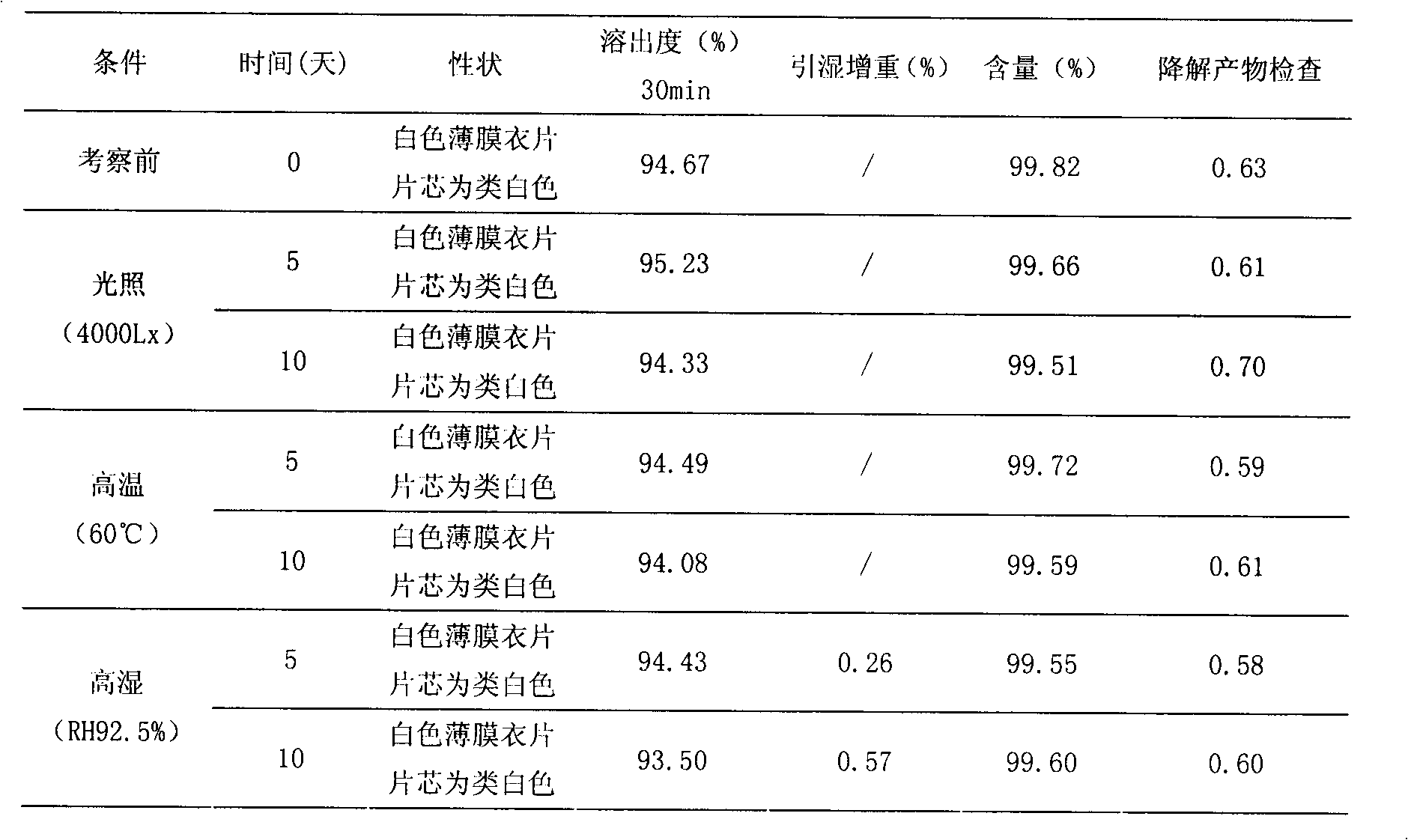
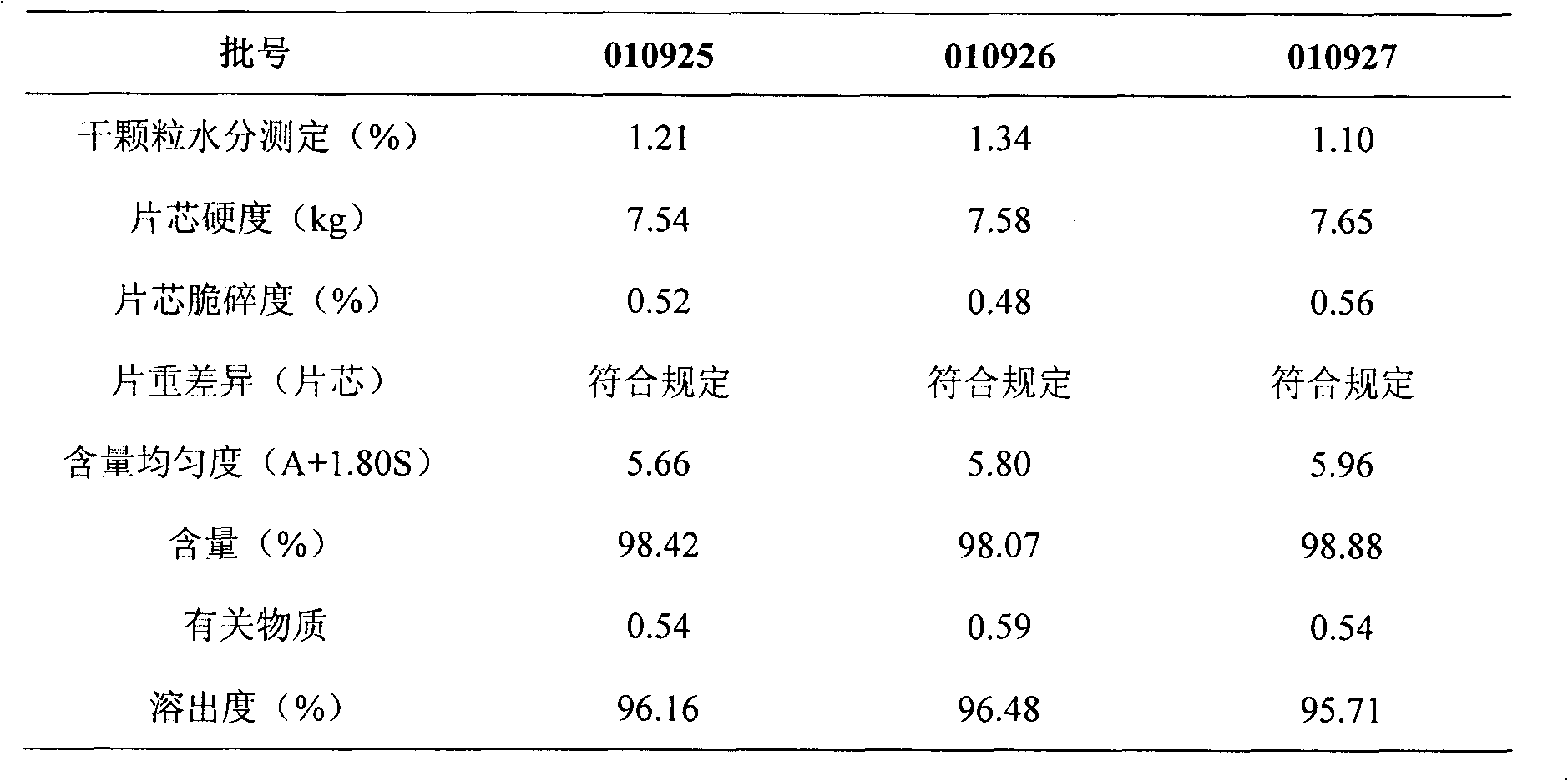
Patents
Literature
89 results about "Sodium Starch Glycolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A kind of levamlodipine besylate crystal, its preparation method and a new pharmaceutical composition containing the crystal
ActiveCN102276516AImprove solubilityGood curative effectOrganic active ingredientsOrganic chemistrySolubilityMedicine
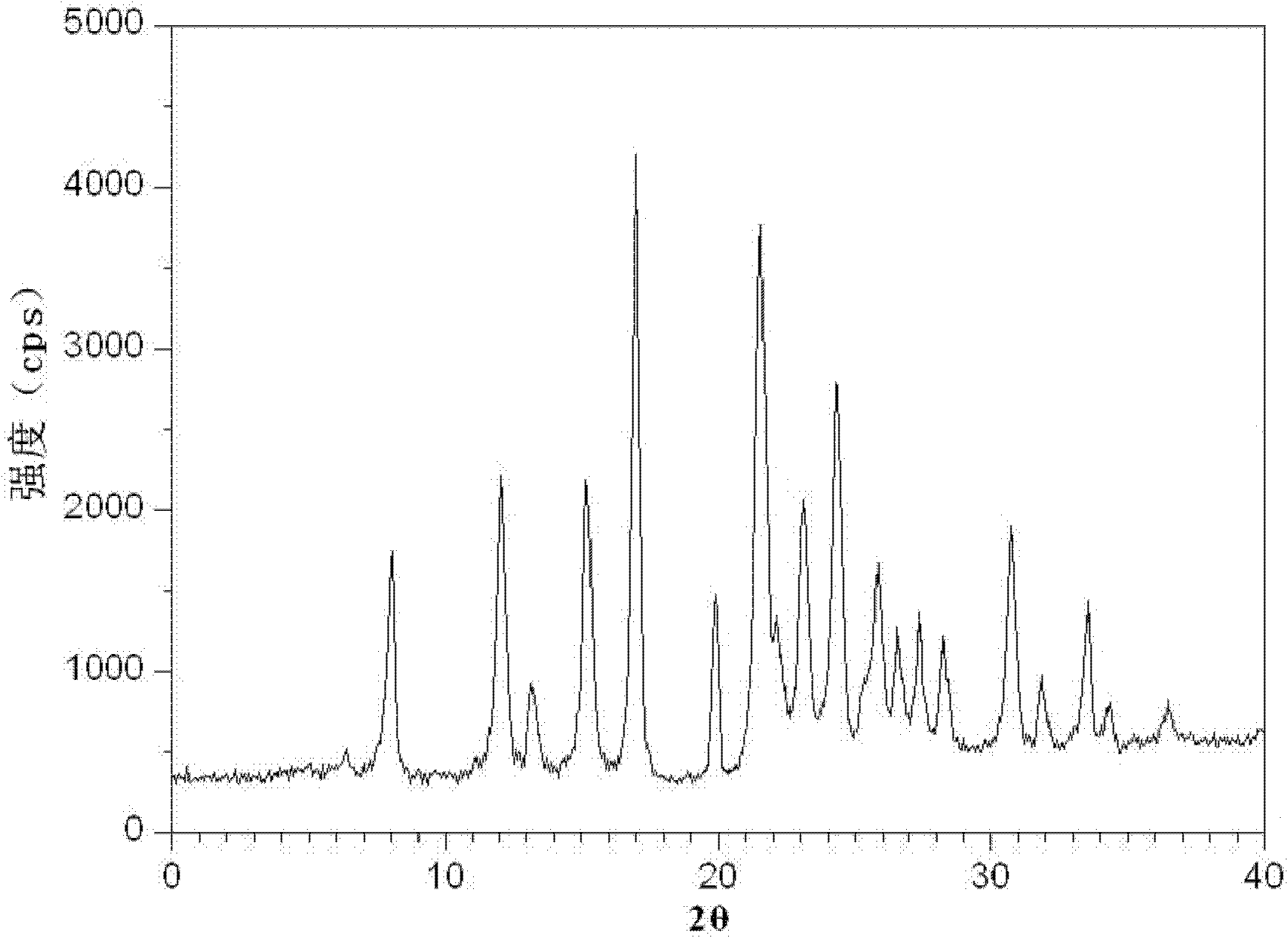
The invention relates to levamlodipine besylate crystals, a preparation method thereof and a brand-new medicinal composition containing the crystals. Characteristic peaks in an X-ray powder diffraction pattern obtained by measuring the levamlodipine besylate crystals by using Cu-K alpha rays are displayed as 8.0, 12.1, 15.4, 17.0, 19.8, 21.6, 23.0, 24.3, 25.7, 27.4, 30.7 and 33.5 degrees at 2 theta. The medicinal composition comprises the levamlodipine besylate crystals and pharmaceutically acceptable excipients, wherein the pharmaceutically acceptable excipients are microcrystalline cellulose, sodium starch glycolate and magnesium stearate. The crystals improve the dissolubility of levamlodipine besylate; and tablets prepared from the crystals have improved bioavailability.
Owner:HAINAN JINRUI PHARMA
Oral Preparation Having Improved Bioavailability
InactiveUS20070254930A1Improve bioavailabilityPowder deliveryBiocideCarmellose CalciumAlkaline earth metal
The present invention relates to an oral preparation of N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl) phenoxy]pentoxyl-benzamidine having improved bioavailability. More particularly, the present invention relates to an oral preparation comprising: N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl) phenoxy]pentoxy}-benzamidine or pharmaceutically acceptable salt thereof; and one or more carbonates selected from the group consisting of alkalimetal carbonate, alkalimetal bicarbonate and alkaline earth metal carbonate, and / or one or more disintegrants selected from the group consisting of sodium starch glycolate, carmellose calcium and croscarmellose sodium. The oral preparation according to the present invention inhibits gelation of N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl) phenoxy]pentoxy}-benzamidine or pharmaceutically acceptable salt thereof in the early stage of release, which increases dissolution rate and remarkably raises bioavailability.
Owner:DONG WHA PHARM CO LTD
Absorbable composite styptic powder and preparation method thereof
The invention discloses absorbable composite styptic powder which is prepared from the following raw materials in parts by weight: 10-30 parts of carboxymethyl chitosan, 10-30 parts of sodium alginate, 10-20 parts of sodium carboxymethylcellulose, 15-50 parts of sodium starch glycolate, 30-60 parts of mannitol, 1-5 parts of calcium chloride and 2,000-4,000 parts of distilled water. The absorbable composite styptic powder disclosed by the invention does not contain any toxic chemical agent and has the functions of inhibiting bacteria, promoting wound healing and inhibiting scar formation; after the absorbable composite styptic powder is scattered and applied, moisture in blood can be uniformly and quickly absorbed by the absorbable composite styptic powder, and a 'white core' is not formed, therefore, the absorbable composite styptic powder is styptic powder which is convenient to use, high in efficiency and extremely good in styptic effect and has no side effect.
Owner:广西达庆生物科技有限公司 +1
Citicoline sodium tablets and preparation method thereof
ActiveCN102028664AIncrease productionLow costOrganic active ingredientsNervous disorderNervous systemCiticoline sodium
The invention provides citicoline sodium tablets which are medicinal preparations for treating sequela of a nervous system caused by a craniocerebral injury or a cerebrovascular accident, and a preparation method thereof. Every 1,000 tablets comprise 100.0 to 300.0g of citicoline sodium, 20.0 to 70.0g of starch, 50.0 to 160.0g of microcrystalline cellulose, 0.6 to 3g of hydroxypropyl methyl cellulose, 1.7 to 5.5g of magnesium stearate, 0 to 10g of sodium starch glycolate and 0 to 6.0g of pregelatinized starch. The preparation method of the citicoline sodium tablets mainly comprises the steps of sieving, weighing, proportioning, premixing, preparing a soft material, preparing wet granules, drying, granulating, mixing, tabletting, performing aluminum-plastic-aluminum packaging and outer packaging and the like. The tablets prepared by the method have the advantages of high yield, low cost, accurate divided dose, relatively stable medicinal physicochemical property and longer storage period and are convenient to carry and use.
Owner:四川梓橦宫药业股份有限公司
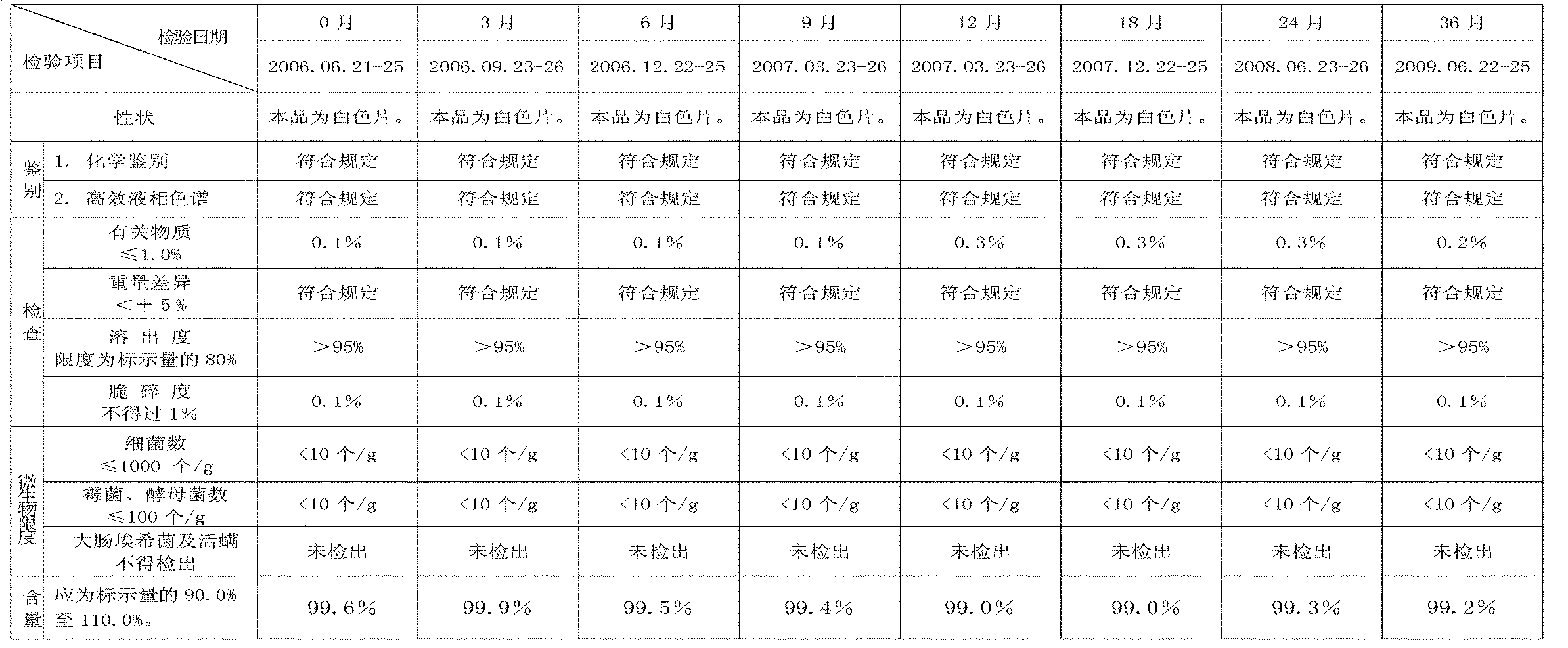
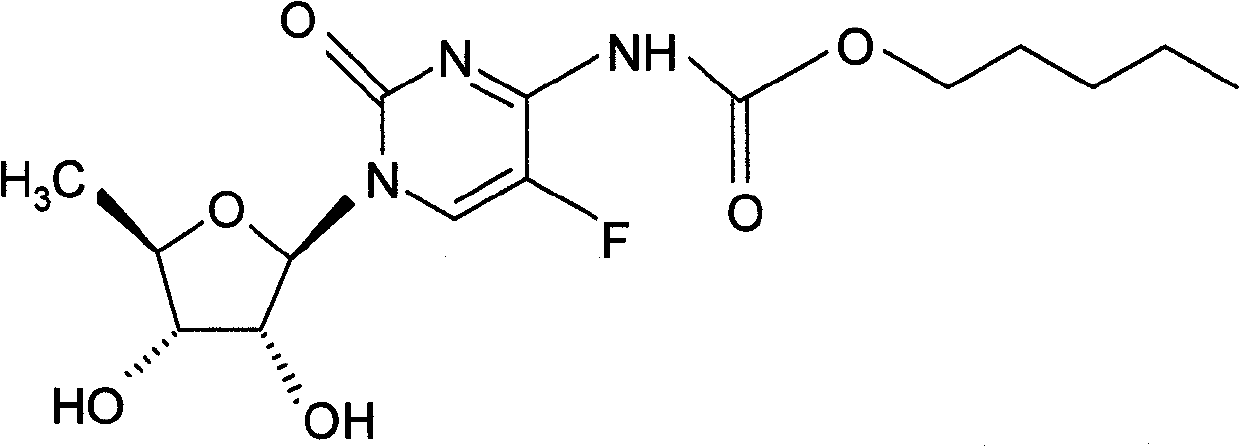
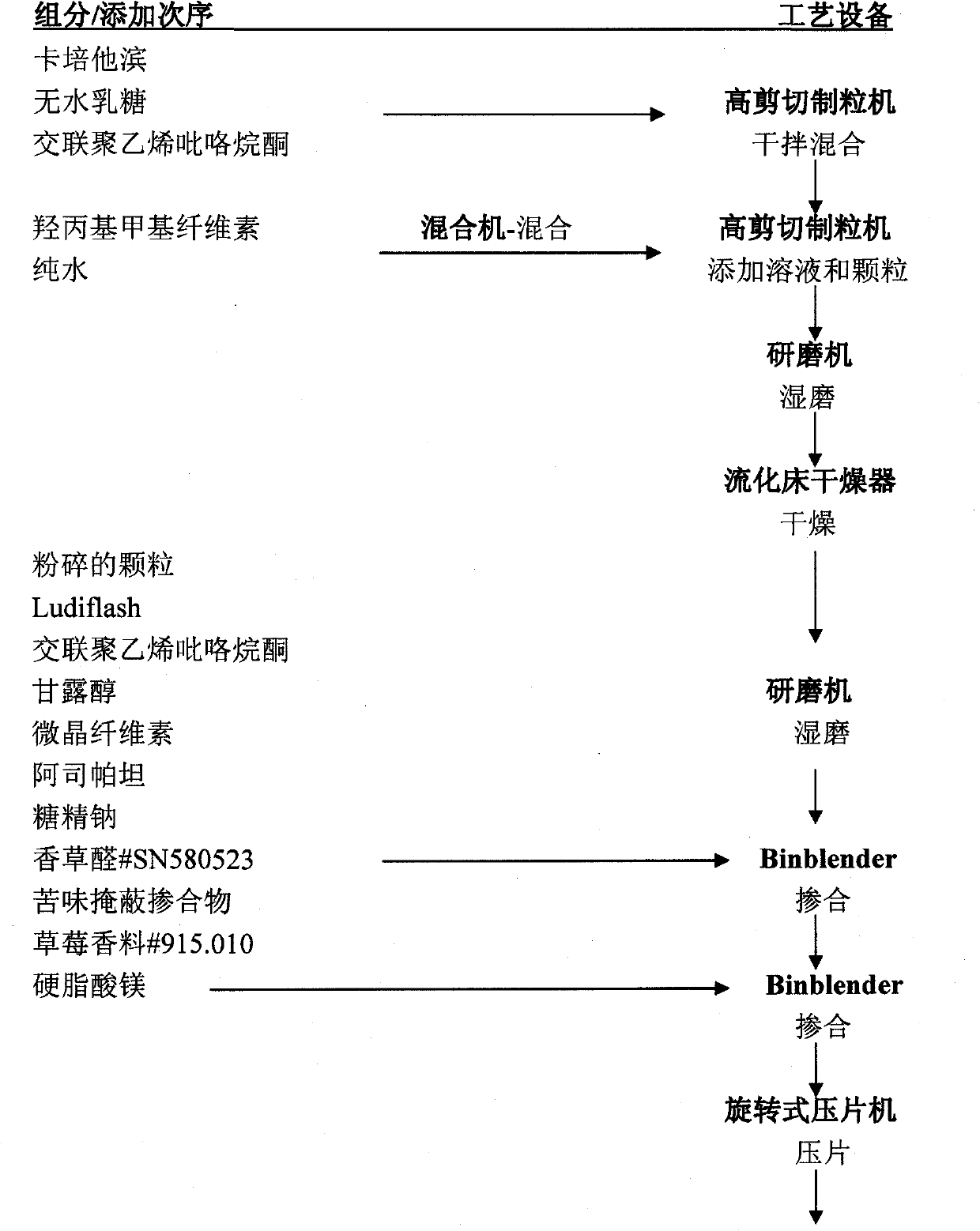
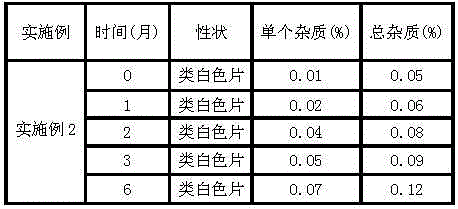
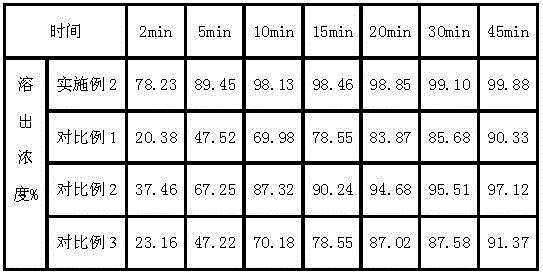
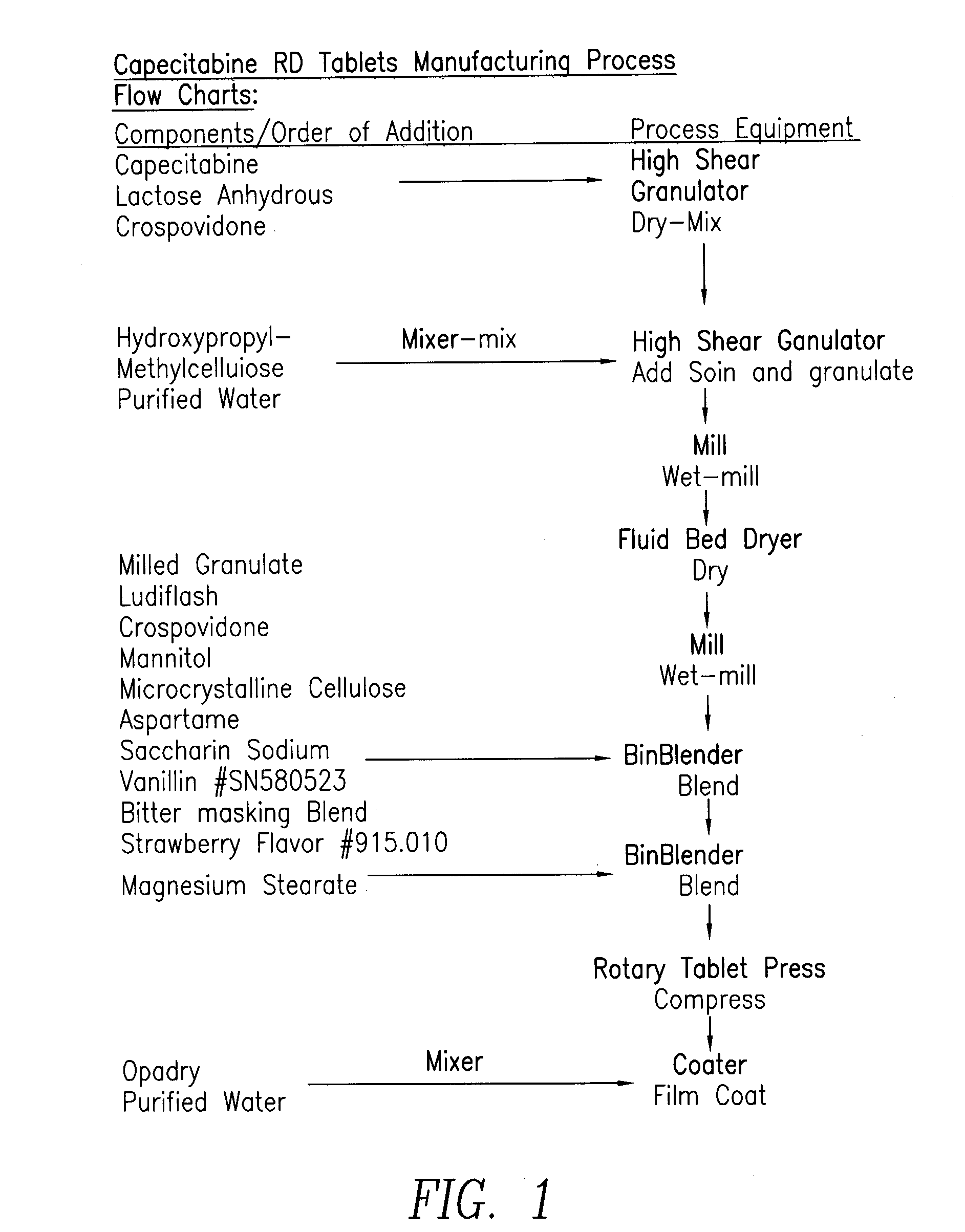
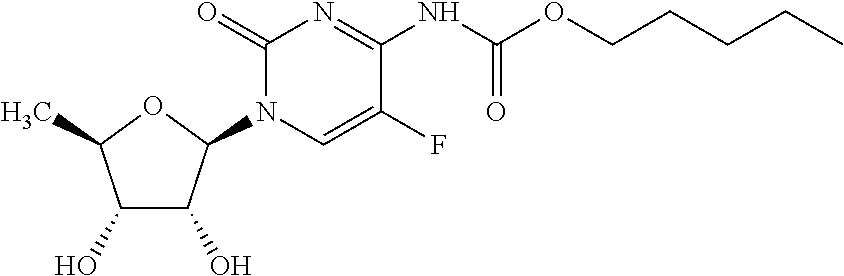
Capecitabine rapidly disintegrating tablets
InactiveCN102369002AOrganic active ingredientsDispersion deliveryCroscarmellose sodiumCarboxymethylcellulose Sodium
There is provided a film coated pharmaceutical composition comprising 5 '-deoxy-5-fluoro-N-[(pentyloxy)-carbonyl]-cytidine (capecitabine) and at least one disintegrant selected from the group comprising of crospovidone (particle size < 15-400 [mu]), croscarmellose sodium, sodium starch glycolate, low-substituted hydroxypropylcellulose, Ludiflash TM or any combination of these, together with other pharmaceutically acceptable excipients to form a rapidly disintegrating tablet.
Owner:F HOFFMANN LA ROCHE & CO AG
Collagen calcium tablet
The invention relates to a collagen calcium tablet, which is a film coated tablet prepared from calcium carbonate, collagen powder, chondroitin sulfuric acid, microcrystalline cellulose, sodium starch glycolate, magnesium stearate and a film coating agent in a certain weight proportion. The collagen calcium tablet product takes the calcium carbonate as a calcium supplement source, the collage powder is added for promoting calcium absorption and bone cell growth, the chondroitin sulfuric acid is matched to promote bone cell growth, resist inflammation and protect bone joints, and the calcium carbonate, the collagen powder and the chondroitin sulfuric acid are compatible with other auxiliary materials; and the product is scientific, reasonable and safe in formula design and suitable for preventing, improving and treating osteoporosis of the old, and has a health care effect of increasing the bone density of middle-aged and aged people.
Owner:BY HEALTH CO LTD
A kind of sugar-free Fengreganmao granule and preparation method thereof
The invention relates to sugar-free anemopyretic cold granules and a preparation method thereof. The granules mainly comprise major materials and auxiliary materials, wherein the major materials comprise isatis root, weeping forsythia, mint, schizonepeta spike, mulberry leaf, rehmannia root, great burdock achene, chrysanthemum, bitter apricot seed, mulberry twig and medicated leaven; and the auxiliary materials mainly comprise stevioside, aspartame, sodium starch glycolate and an appropriate amount of malto dextrin. The preparation method comprises the following steps of: extracting volatile oil from mint and schizonepeta spike for later use; dosing decoction dregs left after the extraction of volatile oil and other major materials in a specified sequence; decocting three times; filtering; concentrating the filtrate at reduced pressure to obtain clear paste of which the relative density is 1.05-1.15 (60 DEG C); performing spray drying to obtain extract powder; adding the screened auxiliary materials into the extract powder; putting the mixture into a dry type granulator to obtain granules of 10-30 meshes; spraying the extracted volatile oil; mixing uniformly; and packaging. The granules prepared with the method have the advantages of no containing of sugar cane, small using quantities of auxiliary materials, greatly lowered dosing amount compared with the original granules, low production cost and high content of arctiin serving as an active ingredient after process improvement.
Owner:GUANGDONG YIFANG PHARMA
Letrozole tablet and preparation method thereof
ActiveCN103356495AImprove stabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsMagnesium stearateLactose
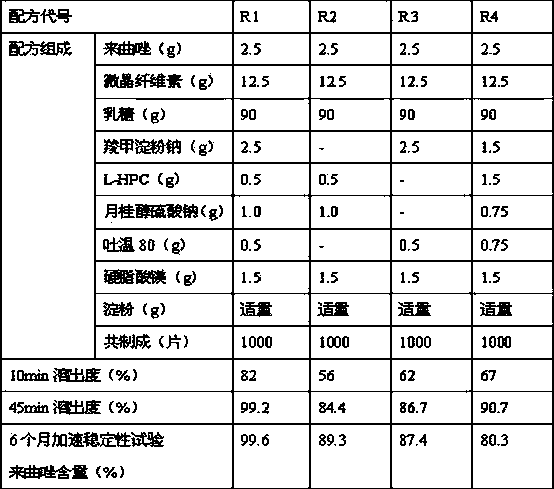
The invention relates to a letrozole tablet and a preparation method thereof. The letrozole tablet comprises the following main auxiliary materials: microcrystalline cellulose, lactose, sodium starch glycolate, L-HPC (low-substituted hydroxypropyl cellulose), sodium lauryl sulfate, Tween 80, magnesium stearate and starch; the preferable sodium lauryl sulfate:Tween 80 ratio is 2:1, and the sodium starch glycolate:L-HPC ratio is 5:1. The tablet has stable product quality, obviously enhances the letrozole dissolution, and effectively enhances the bioavailability; and the tablet preparation technique is simple, does not need special equipment, and is convenient to operate and suitable for industrial production.
Owner:HAINAN LINHENG PHARMA
Ibuprofen and narcotic analgesic compositions
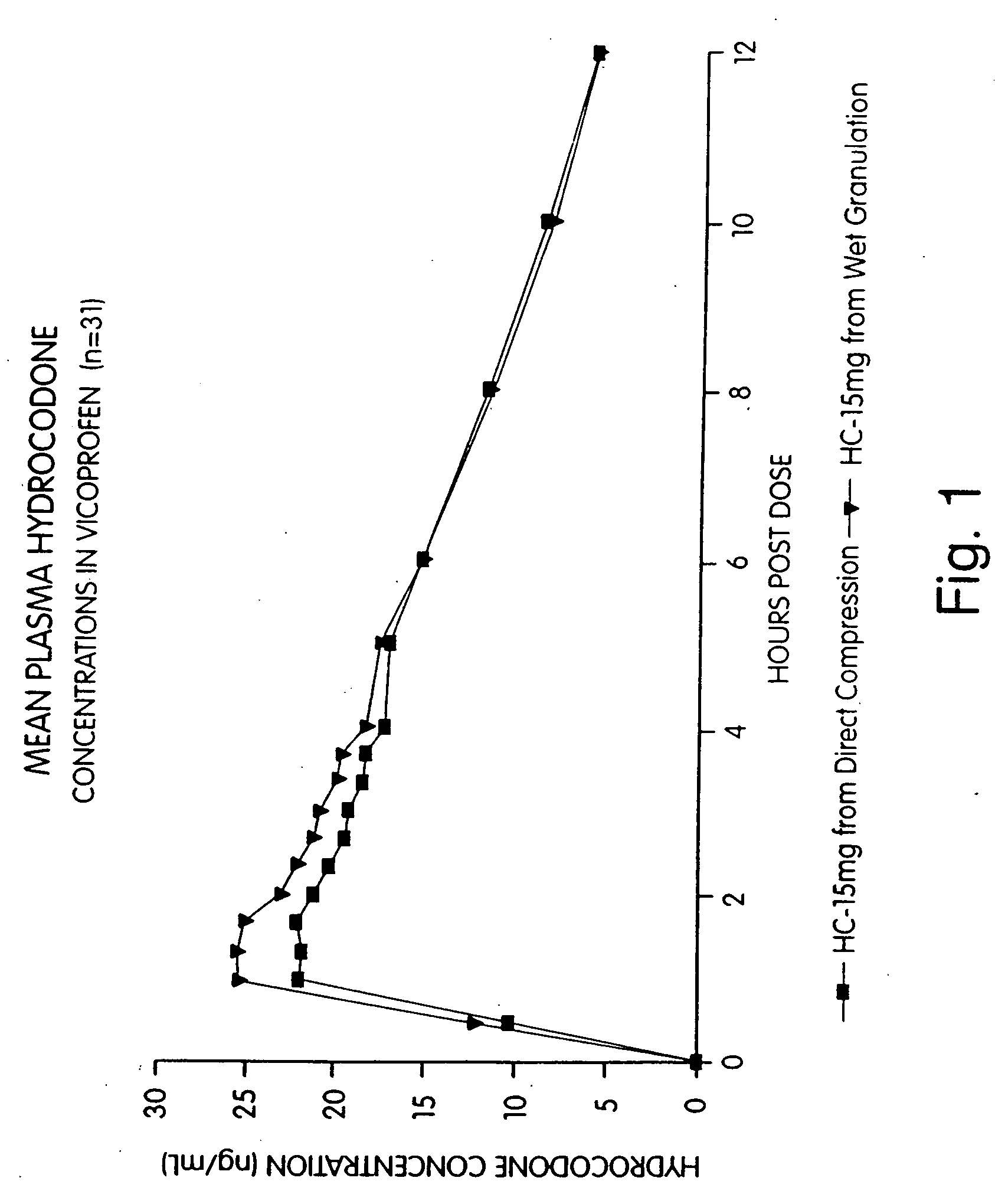
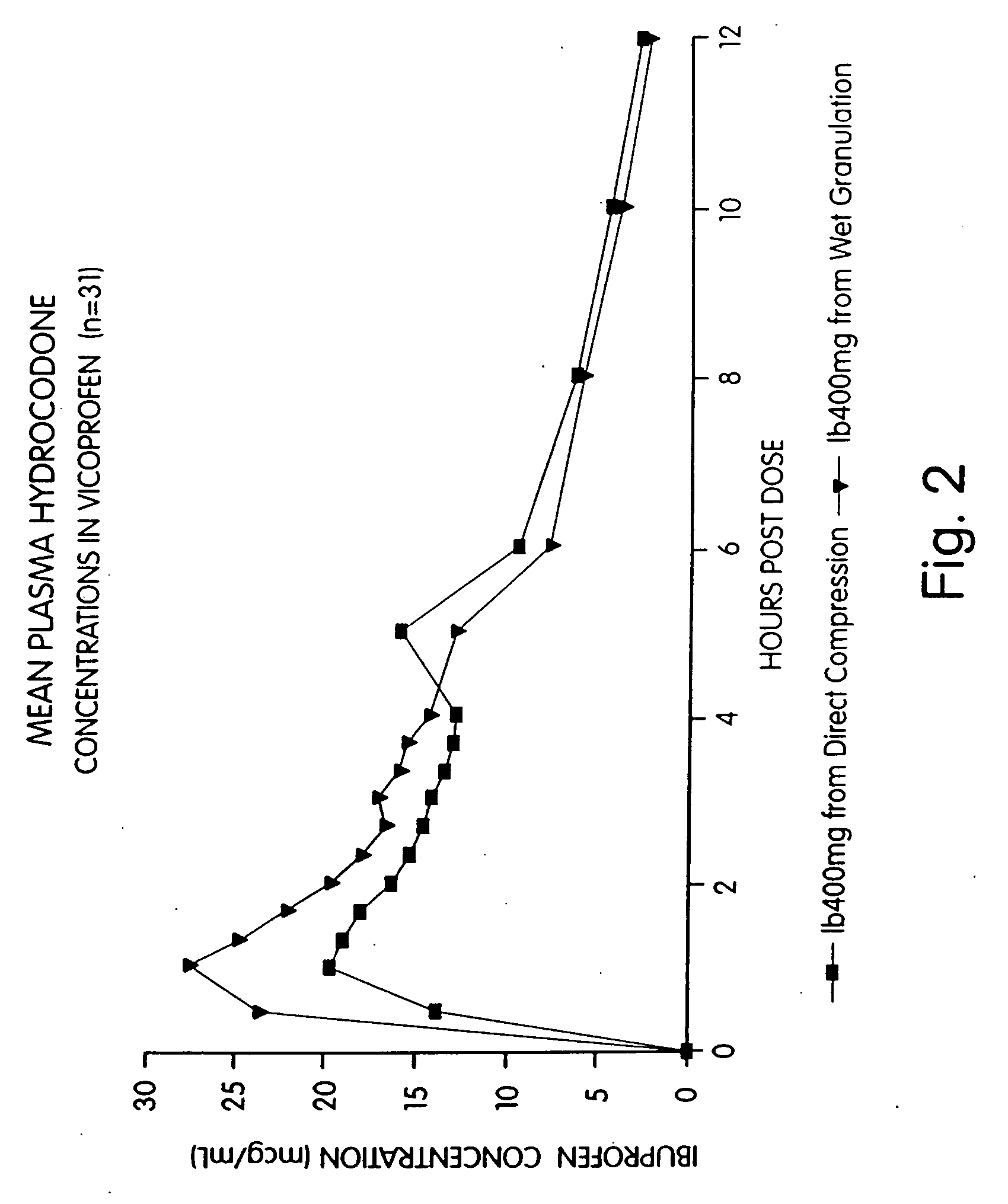
Provided herein are compositions and methods of making compositions of ibuprofen in combination with a narcotic analgesic. Specifically provided is a pharmaceutical tablet composition comprising ibuprofen; a narcotic analgesic; colloidal silicon dioxide; a filler selected from the group consisting of microcrystalline cellulose and powdered cellulose; a disintegrant selected from the group consisting of croscarmellose sodium, crospovidone, and sodium starch glycolate; a binder consisting of an akylhydroxy methylcellulose; a starch; and a lubricant. Also provided herein is a method of preparing a pharmaceutical tablet composition comprising: (a) Granulating ibuprofen, a narcotic analgesic, a first glidant, a first disintegrant, a binder, and starch to form granules wherein said granulating step comprises a wet granulation process; (b) blending the granules with extra-granular material comprised of a second glidant, a second disintegrant, a filler and starch to form a blend of granules and extra-granular material; and (c) compressing the blend into a tablet.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Glimepiride tablet and preparation method thereof
ActiveCN102488667BImprove hydrophilicityReduce dosageMetabolism disorderSulfonylurea active ingredientsGlimepiridumStatistical analysis
Owner:CHONGQING CONQUER PHARML
Lafutidine coated tablet and preparation method thereof
InactiveCN102688217AOrganic active ingredientsDigestive systemSodium Starch GlycolatePharmaceutical formulation
The invention relates to the field of a medicinal preparation, in particular to a lafutidine-containing coated tablet and a preparation method thereof.. The preparation provided by the invention is an oral solid preparation consisting of lafutidine, lactose, microcrystalline cellulose, starch, sodium starch glycolate and magnesium stearate. In order to ensure the main dissolution rate, most of the auxiliaries in the prescription are hydrophilic. In the invention, the technology of the preparation is simple and adopts a few auxiliaries; and the product has stable quality and good dissolution rate, takes an effect quickly and acts for a long time.
Owner:JIANGSU RUNBANG PHARMA
Nanometer preparation of strong rooting agent, preparation method and application thereof
InactiveCN102524261AEasy to prepareEasy to operateBiocidePlant growth regulatorsDistilled waterSodium Starch Glycolate
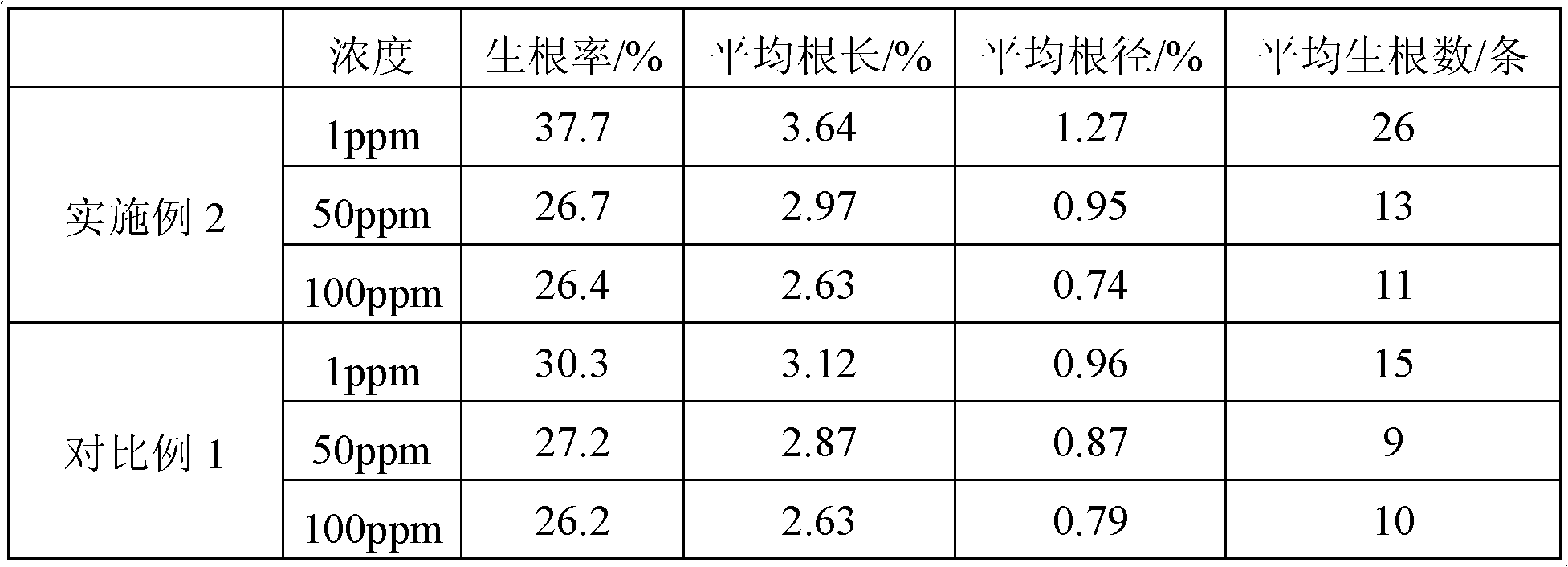
Provided are a nanometer preparation of a strong rooting agent, a preparation method and application thereof. The preparation method of the nanometer preparation of the strong rooting agent is characterized by comprising the following steps: (1) dissolving the strong rooting agent of 50mg in distilled water of 100ml; (2) adding a surfactant of 10mg and a disintegrating agent of 20mg, mixing for 0.5h, enabling the surfactant to be sodium dodecyl sulfate, and enabling the disintegrating agent to be sodium starch glycolate; and (3) performing ultrasound for 1h. The preparation method of the nanometer preparation of the strong rooting agent is simple and convenient to operate, raw materials can be obtained easily, and the prepared nanometer preparation of the strong rooting agent is nano-scale particles so that the nanometer preparation can come into play at the nanometer level. Besides, the nanometer preparation of the strong rooting agent has remarkable effects on rooting of a chlorophytum comosum plant.
Owner:LIAONING NORMAL UNIVERSITY
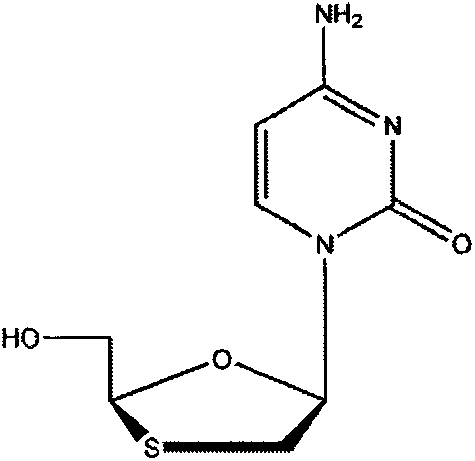
Lamivudine tablet composition and preparation method thereof
InactiveCN107303282ADelicate appearanceSmooth appearanceOrganic active ingredientsDigestive systemSilicon dioxideSodium Starch Glycolate
The invention relates to a lamivudine tablet composition and a preparation method thereof. The core of the lamivudine tablet composition is prepared from the following ingredients: 80-120 parts of lamivudine, microcrystalline cellulose 90-150 parts, 4-20 parts of sodium starch glycolate, 1-6 parts of silicon dioxide, 0.5-4 parts of magnesium stearate. The preparation method is as follows: sieving; weighing lamivudine, microcrystalline cellulose, sodium carboxymethyl starch, silicon dioxide, and magnesium stearate, mixing evenly, measuring the content of the mixed powder, and calculating the tablet weight after passing the test. It is directly pressed into tablets, coated, dried, fully inspected and packaged to obtain the finished product.
Owner:南京清洛生物科技有限公司
Glimepiride tablet and preparation method thereof
ActiveCN102488667AImprove hydrophilicityReduce dosageMetabolism disorderSulfonylurea active ingredientsGlimepiridumStatistical analysis
The invention discloses a Glimepiride tablet, wherein the Glimepiride tablet comprises the following components: Glimepiride tablet bulk pharmaceutical chemical, lactose, sodium starch glycolate, microcrystalline cellulose, microcrystalline silica gel and magnesium stearate at the weight ratio of (2-6):(60-90):(10-30):(10-60):(2-6):1, wherein the Glimepiride tablet bulk pharmaceutical chemical ispowder with the particle size less than 2 mu m. The invention also discloses a preparation method. With regard to Glimepiride, pharmaceuticals are smashed into particulates with the particle size less than 2mu m by an improved technology, and the hydrophilicity of the pharmaceuticals is improved; and the problem that the dissolution velocity of the pharmaceuticals is influenced is solved. Statistical analysis indicates that the average dissolution degree is above 98.5% by adopting the improved prescription and technology, and the accelerated test result for 6 months is very stable; and the research on primary pharmacokinetic parameters indicate that the preparation has the advantages of better dynamic course in human bodies and better absorption elimination properties through the checkingof statistics.
Owner:CHONGQING CONQUER PHARML
Cefteram pivoxil crystal and preparation method thereof and composition tablets containing crystal
ActiveCN102351886AHigh dissolution rateImprove bioavailabilityAntibacterial agentsOrganic active ingredientsCelluloseActive agent
The invention relates to a cefteram pivoxil crystal and a preparation method thereof and composition tablets containing the crystal. The grain diameter of the crystal is 1 to 5 microns; and the composition tablets of the crystal are obtained by coating tablets prepared from 30 to 120 grams of cefteram pivoxil crystal, 15 to 65 grams of starch, 5 to 20 grams of hydroxypropyl cellulose, 10 to 45 grams of sodium starch glycolate, 0.1 to 2 grams of magnesium stearate and a proper amount of pure water. The crystal with the grain diameter of 1 to 5 microns is prepared by using a method of compounding ultrasonic and micro emulsion; the cefteram pivoxil can be quickly released from a medicament, so that the dissolution rate of the medicament is improved and the bioavailability is improved; by using the method, separation and purification of the cefteram pivoxil and a surfactant can be realized; and because the composition contains the crystal, the dissolution rate of the medicament is improved and the bioavailability is improved.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Compositions for a powder having an aqueous phase
InactiveUS20110311470A1Light and fresh feelingCosmetic preparationsBody powdersCross-linkPersonal care
In an embodiment of the present invention, there is provided a personal care composition having: a) at least one hydrophilic binder chosen from a sodium starch glycolate cross-linked with a phosphate ester group; b) at least one hydrophobic powder filler; and c) water, wherein the composition is in loose powder form. In another embodiment of the present invention, there is provided a method of making a personal care composition involving: a) forming a powder phase, comprising: i) at least one hydrophilic binder chosen from a sodium starch glycolate cross-linked with a phosphate ester group; and ii) at least one hydrophobic powder filler; b) providing an aqueous phase, comprising water; and c) adding the aqueous phase to the powder phase, wherein the personal care composition is in loose powder form.
Owner:LOREAL SA
Elagolix formulation
ActiveUS11273128B1Good storage stabilityReduce conversionOrganic active ingredientsDigestive systemCrospovidonesCroscarmellose sodium
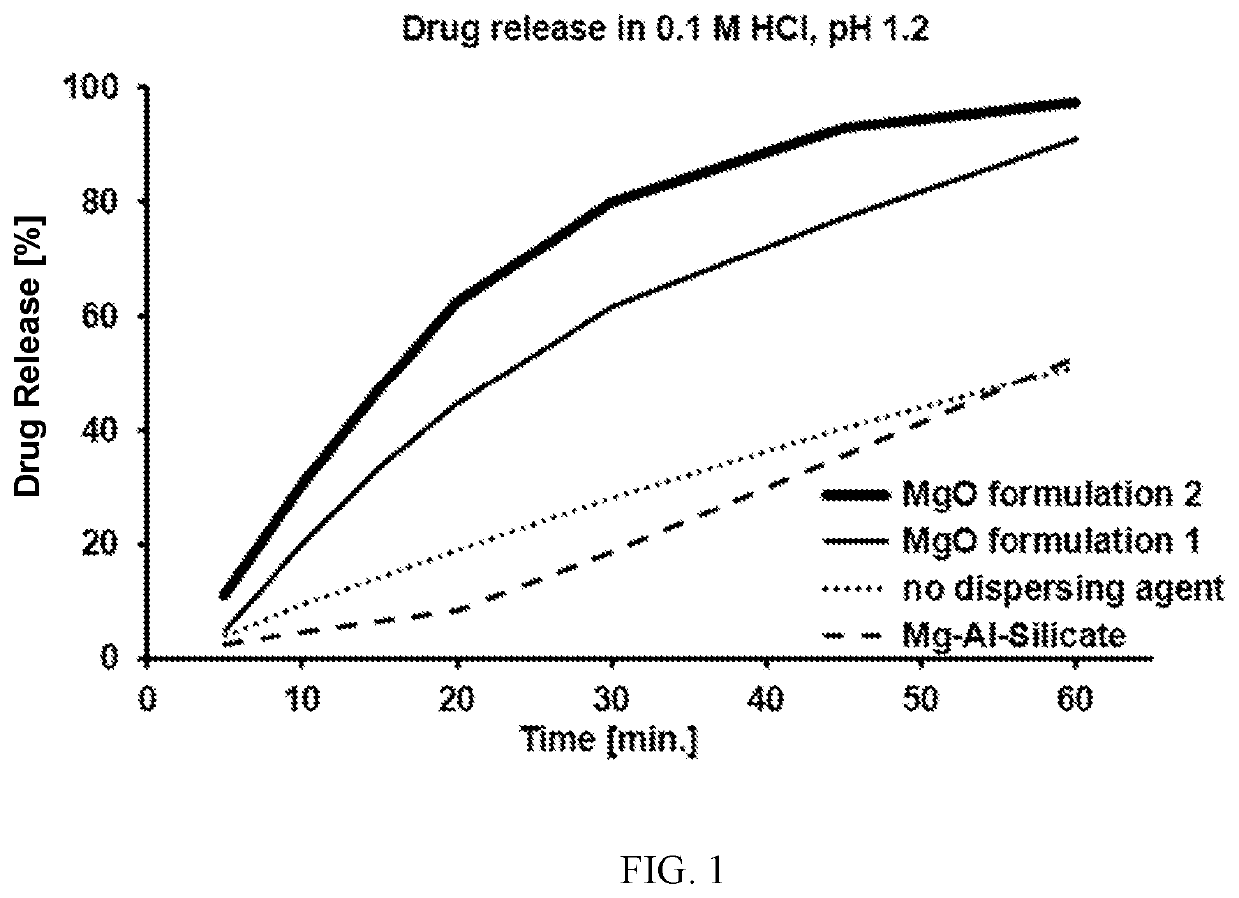
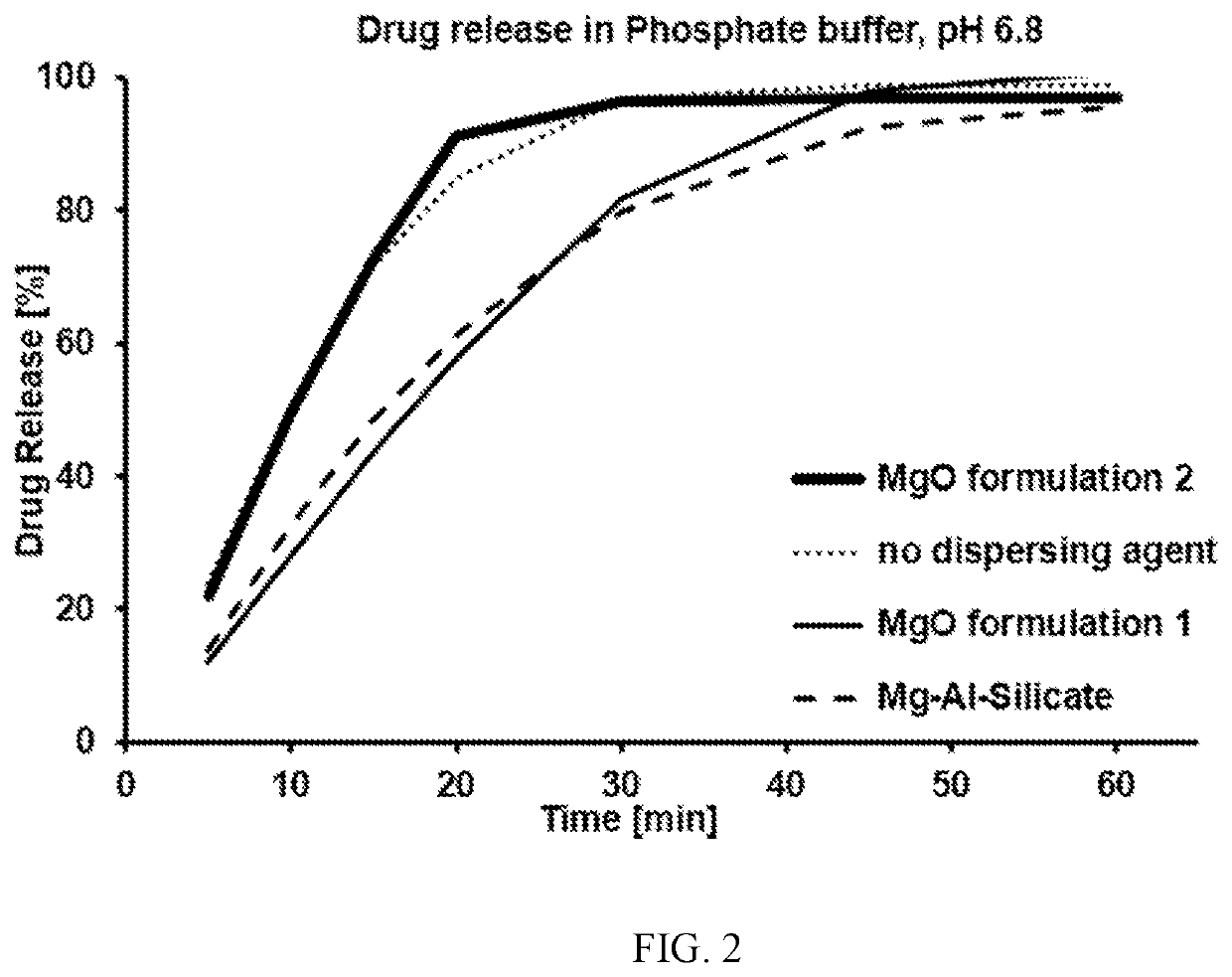
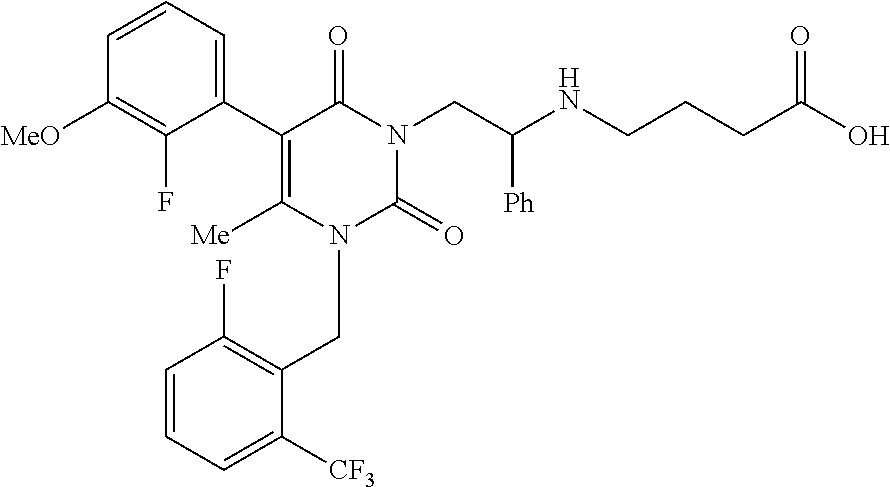
A pharmaceutical formulation is disclosed which includes at least: (1) elagolix sodium, (2) magnesium oxide, and (3) at least one disintegrating agent, such as crospovidone, croscarmellose sodium, sodium starch glycolate, pregelatinized and mixtures thereof. A tablet is also disclosed which includes a tablet core formed from the pharmaceutical formulation. The elagolix sodium tablets of the present disclosure display improved dissolution rates when tested using for example the Tablet Sink Time test. The tablets also exhibit improved storage stability of the elagolix sodium, with a reduction in degradation products during storage.
Owner:SANDOZ AG
Medicine for treating child influenza and preparation method thereof
InactiveCN102499920ASignificant effectImprove securityOrganic active ingredientsAntipyreticPseudoephedrine HydrochlorideMagnesium stearate
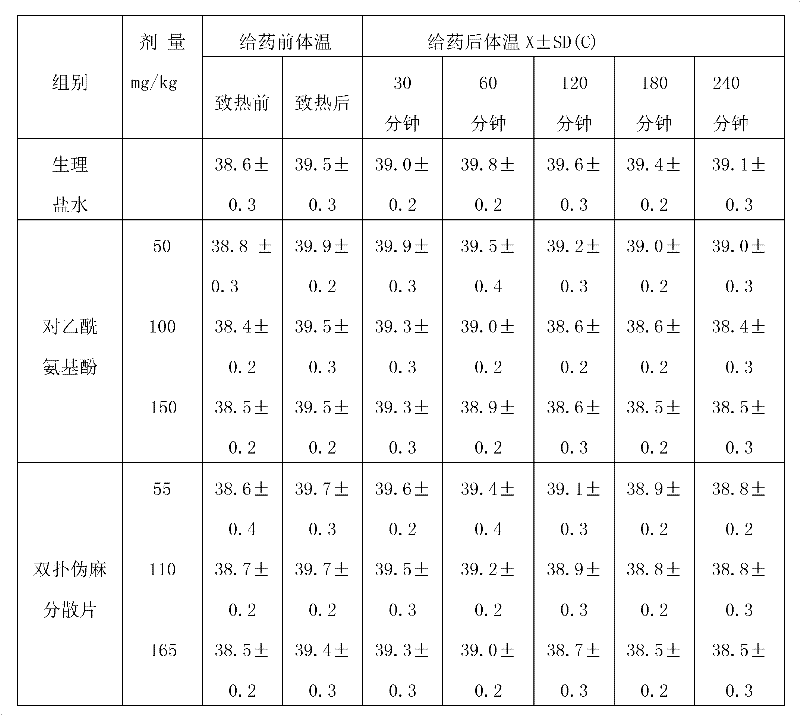
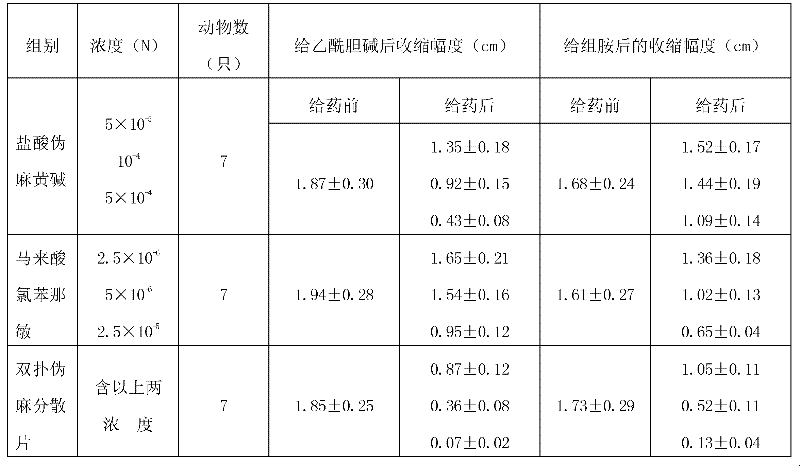
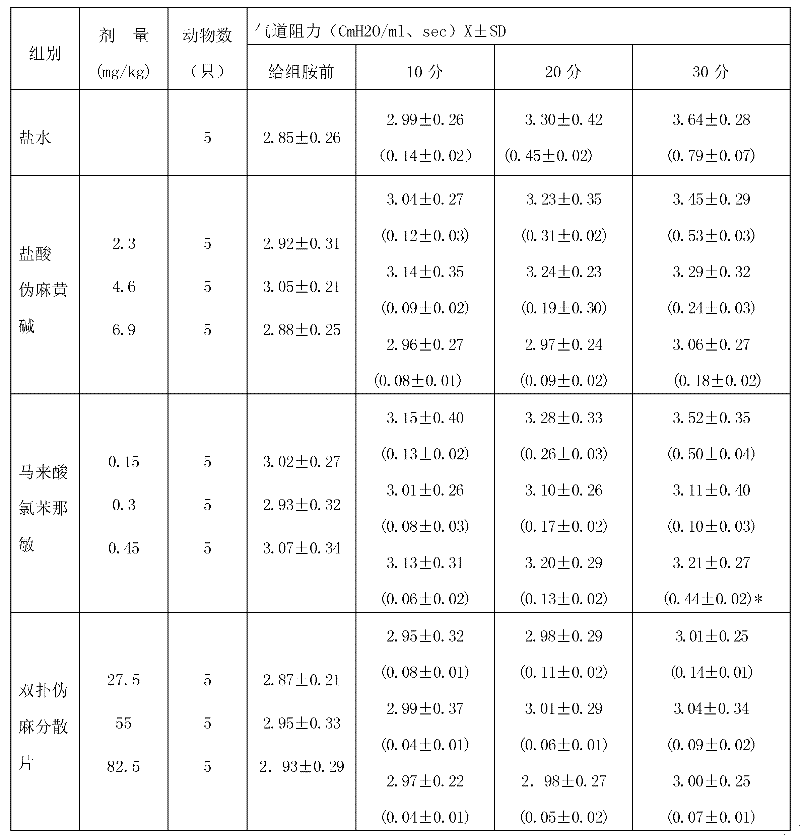
The invention relates to a medicine for treating child influenza, which takes acetaminophen, pseudoephedrine hydrochloride and chlorphenamine maleate as main materials, and takes microcrystalline cellulose, starch, aspartame, sodium starch glycolate, magnesium stearate, 60% ethanol and 2% sodium starch glycolate aqueous solution as auxiliary materials. A preparation method is as follows: the acetaminophen, the pseudoephedrine hydrochloride and the chlorphenamine maleate are crushed into fine powder for spare use; the chlorphenamine maleate is uniformly mixed with the acetaminophen and the pseudoephedrine hydrochloride through a equivalent incremental method; parts of the microcrystalline cellulose, the starch, the aspartame and the sodium starch glycolate are weighed to be uniformly mixed, are crushed to sieve, and are mixed again, the 60% ethanol and the 2% sodium starch glycolate aqueous solution are added to the prepared mixture, are completely stirred to make a soft material, and wet particles are produced after the sieving; the produced wet particles are dried at 75 DEG C and granulated; after the granulation, the residual auxiliary materials of the microcrystalline cellulose, the starch, the sodium starch glycolate and the magnesium stearate are added again to mix uniformly; and dispersible tablets are pressed and packaged. The medicine has the advantages of obvious curative effect, good safety, convenience in medicine taking and suitable taste for children.
Owner:山西皇城相府药业股份有限公司
Glimepiride tablet and preparation method thereof
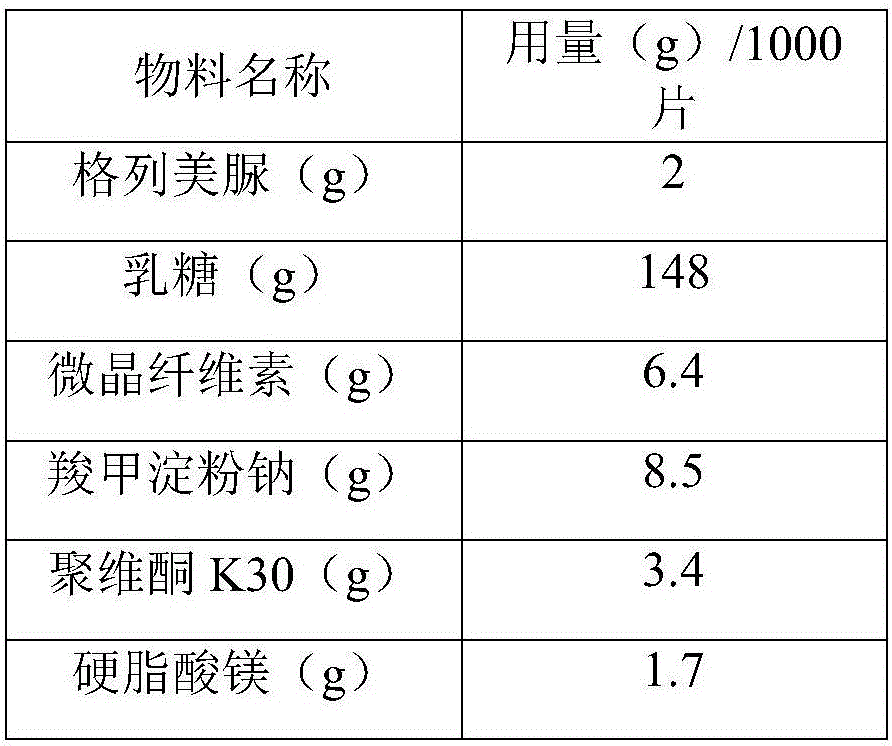
InactiveCN106361712ASmall particle sizeImprove liquidityMetabolism disorderSulfonylurea active ingredientsMedicineCurative effect
The invention relates to a glimepiride tablet and a preparation method thereof, belonging to the technical field of medicine. The glimepiride tablet is prepared from the following components in parts by weight: 1-2 parts of glimepiride, 74-128 parts of lactose, 3.2-6.4 parts of microcrystalline cellulose, 4.25-8.5 parts of sodium starch glycolate, 1.7-3.4 parts of povidone K30 and 0.85-1.7 parts of magnesium stearate. The glimepiride tablet provided by the invention enhances the bioavailability, thereby enhancing the curative effect. The glimepiride tablet has the advantages of higher stability, better leaching effect and higher unit-dosage use efficiency, provides a good option for medicine selection and clinical application in hospitals, and has very high economic and social meanings.
Owner:SHIJIAZHUANG HUAXIN PHARMA
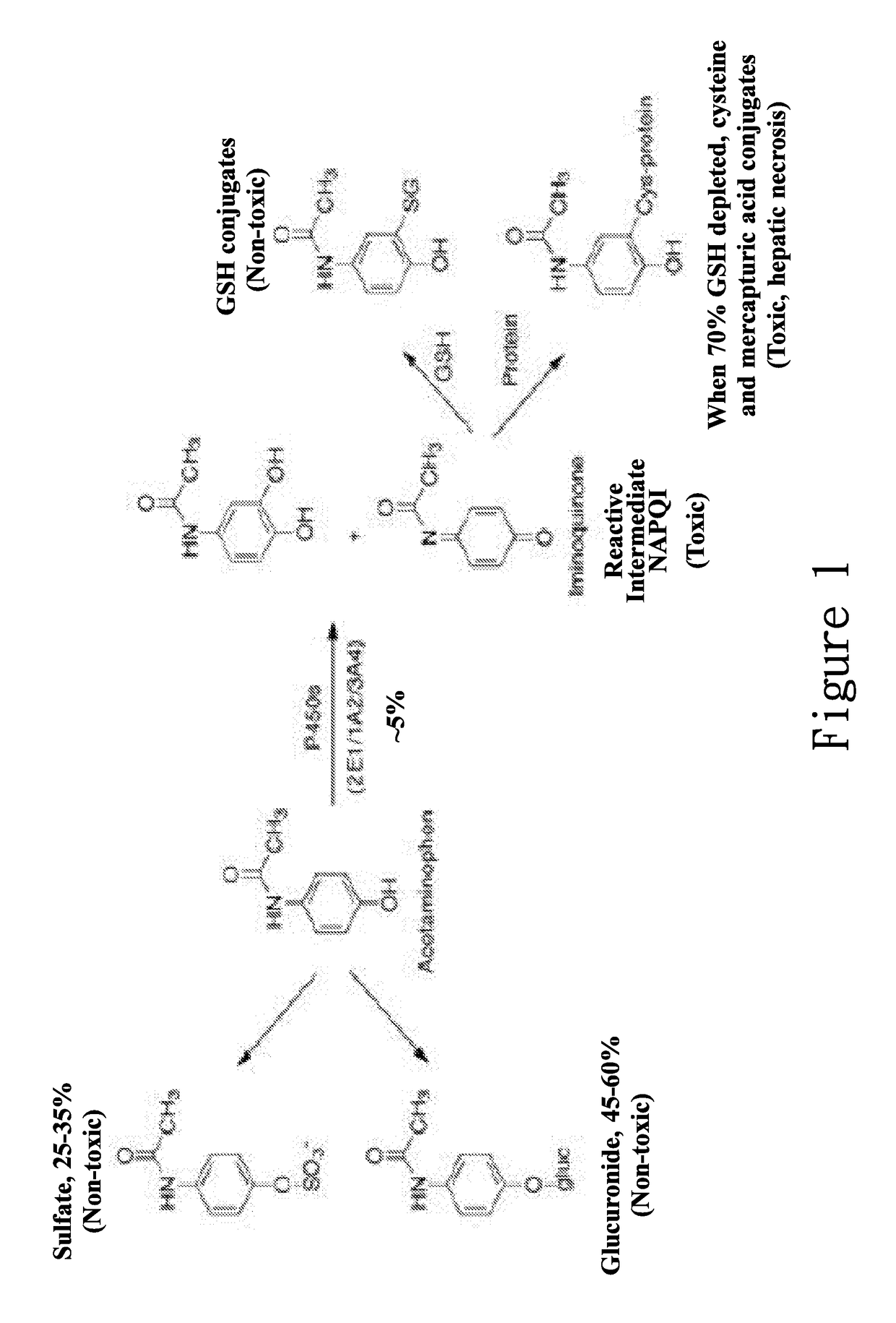
New hepatotoxicity-free pharmaceutical composition containing acetaminophen drugs
ActiveUS20170172950A1Reduce liver toxicityOrganic active ingredientsAntipyreticColloidal silicaPolyoxyethylene castor oil
A new compound composition that is free of a side effect to a liver and used for alleviating the toxicity of an acetaminophen (APAP) medicament to the liver. The compound composition comprises (a) a pharmaceutically effective amount of acetaminophen and (b) a frequently-used safe and pharmaceutically acceptable excipient that can be combined with one or more than two medicaments that can reduce the toxicity of a drug via liver enzyme CYP2E1 metabolism to the liver. The compound is selected from the following group: Tween 20, microcrystalline cellulose, dicalcium phosphate, polyoxyethylene 23 lauryl ether, saccharin, mannitol, polyoxyethylene alkyl ether, sucralose, pyrrolidone, sodium starch glycolate, acrylic resin S100, carboxymethyl cellulose sodium, polyoxyethylene polyoxypropylene, menthol, low-substituted hydrocarbon propyl cellulose, pregelatinized starch, Dextrates NF hydrated, citric acid, polyoxyethylene castor oil, colloidal silica, polyethylene glycol monostearate aliphatic ester, sorbic acid, lemon oil, hydroxypropyl cellulose, sorbitol, acesulfame potassium, hypromellose phthalate, lactose monohydrate, maltodextrin, Brij 58, Brij 76, Tween 80, Tween 40, PEG 400, PEG 4000, PEG 2000, and the like, so as to reduce the side effect of the toxicity caused by acetaminophen to the liver.
Owner:INT EDUCATION FOUND
Medicament for treating dyspepsia and preparation thereof
InactiveCN101209346ASymptoms improvedPromote digestionPowder deliveryPeptide/protein ingredientsAmylaseEthyl hydroxybenzoate
The invention relates to a drug for the treatment of dyspepsia, and takes amylase, hawthorn fluid extract, calcium pantothenate, nicotinamide and vitamin B1 as the active ingredients. The preparation method is that: the hawthorn is prepared into the hawthorn fluid extract and then is dissolved in 40 percent ethanol; the amylase, the calcium pantothenate, the nicotinamide, the vitamin B1, calcium hydrophosphate and microcrystalline cellulose are taken, mixed evenly and screened; the 40 percent ethanol solution which is dissolved with the hawthorn fluid extract is used as the binding agent for granulation, drying and stabilization; the sodium starch glycolate and talc powder are added and mixed evenly for granule preparation and tablet pressing; or the prescription dose of sodium benzoate, ethyl hydroxybenzoate, hawthorn fluid extract and caramel colorant are taken to be dissolved in purified water, the steam is used for heating till boiling for 30 minutes, and then the mixture is cooled slightly for standby; the prescription dose of calcium pantothenate, nicotinamide, vitamin B1 and edible flavor are dissolved in the purified water; the solution is merged and cooled; the prescription dose of amylase is taken, dissolved in purified water and screened; the solution is merged, the purified water is added till the full amount, and the oral solution is obtained by evenly stirring and filtration.
Owner:GUANGDONG ZHONGSHENG PHARMA
Medicinal tadalafil composition tablets for treating urological diseases
InactiveCN105193749AGood water solubilityLow impurity contentOrganic active ingredientsOrganic chemistryTadalafilMagnesium stearate
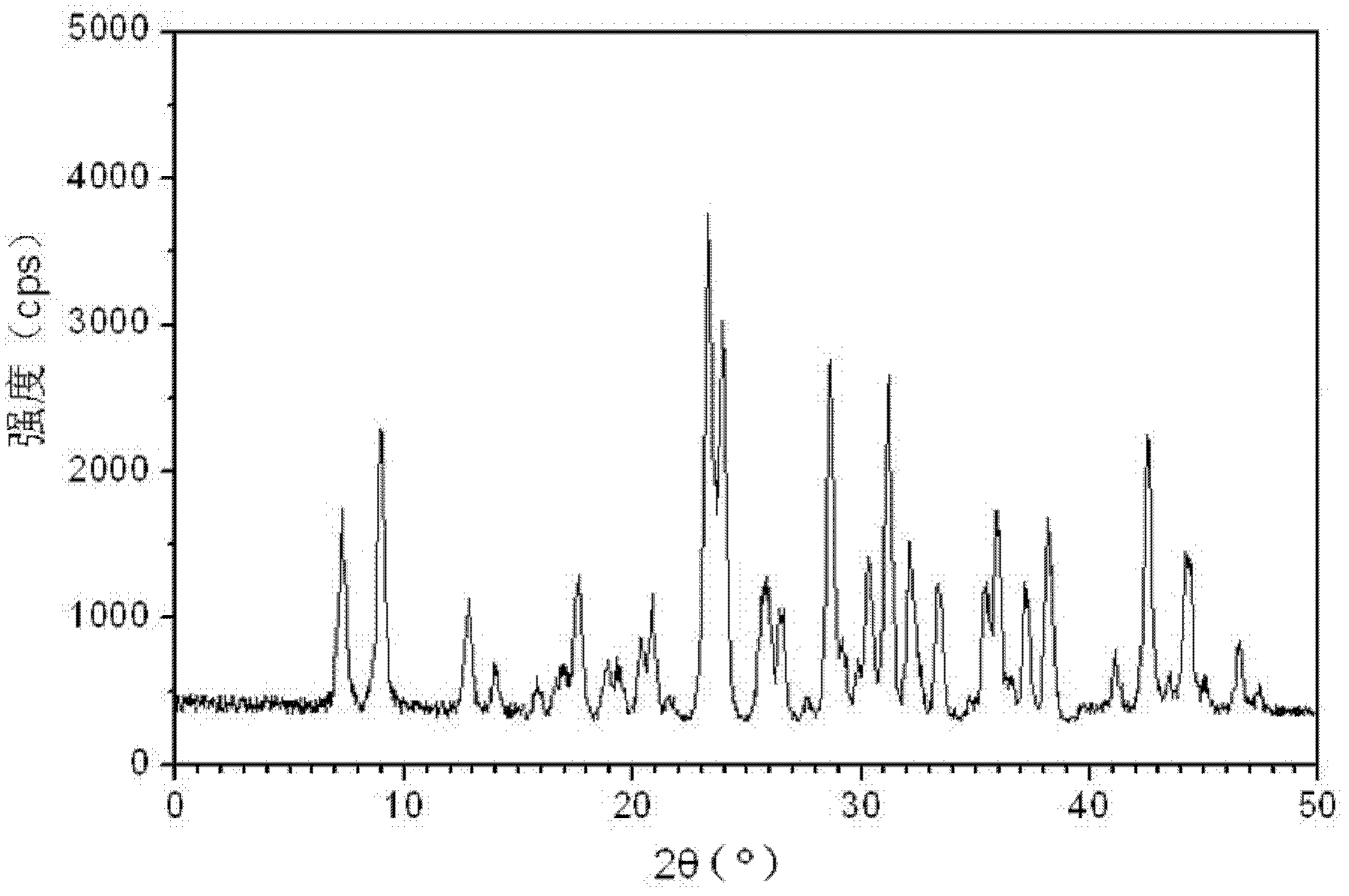
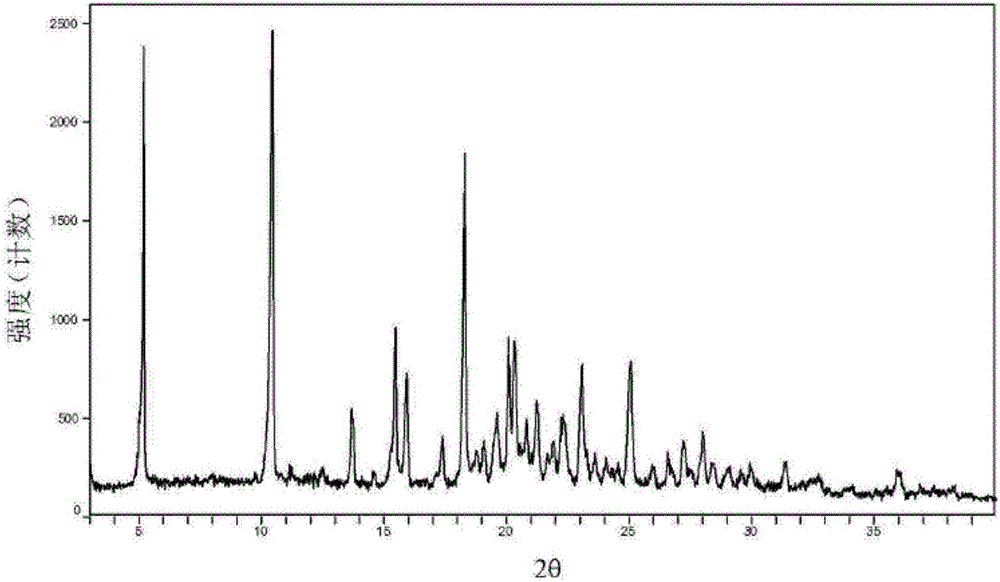
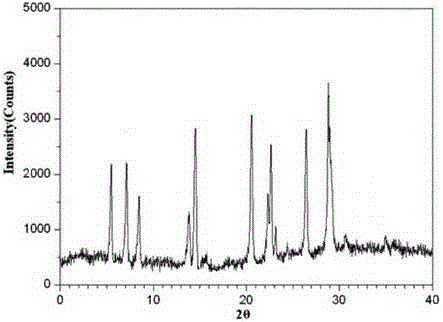
The invention relates to medicinal tadalafil composition tablets for treating urological diseases, and belongs to the technical field of medicine. The tablets are prepared from an internally added original accessory, an adhesive and a lubricant, wherein the internally added original accessory consists of tadalafil, sodium starch glycolate, starch, lactose and microcrystalline cellulose; the adhesive is prepared from sodium dodecyl sulfate and purified water; the lubricant is magnesium stearate. The tadalafil is a compound of a novel crystal form, an X-ray powder diffraction pattern measured by Cu-K alpha rays is shown in picture 1, the tadalafil is different from that reported in the prior art, and a test shows that the compound of the novel crystal form has obviously improved water solubility, low impurity content and high stability; compared with the prior art, the tablets prepared from the tadalafil compound of the novel crystal form are high in dissolubility and stability and low in impurity content, and safety in clinical application is improved.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Adhesive agent used for environmental and low-cost paper boxes and preparation method thereof
InactiveCN103333646AImprove adhesionNo pollution in the processNon-macromolecular adhesive additivesStarch derivtive adhesivesPentachlorophenolSodium metasilicate
The invention discloses an adhesive agent used for environmental and low-cost paper boxes and a preparation method thereof. The adhesive agent comprises following components: acrylic acid emulsion, starch, titanium dioxide, sodium silicate, sodium metasilicate, sodium citrate, ammonium persulfate, vinyltriethoxysilane, phthalic anhydride, sodium pentachlorophenol, zine stearate, sodium starch glycolate, and water. The adhesive agent used for environmental and low-cost paper boxes adopts acrylic acid emulsion and starch as the main materials, through oxidation treatment of starch, the performance of the starch is improved, and the adhesivity of the product is strengthened. Furthermore, the product is environmental and pollution free, preparation method is simple, the cost is low, and the product is suitable for mass production.
Owner:ANHUI KING AUTO ELECTRONICS TECH CO LTD
Compound allopurinol dispersible tablet
InactiveCN101766627AImprove solubilityIncrease release speedOrganic active ingredientsSkeletal disorderAdhesiveSilica gel
The invention relates to the field of medicines, in particular to a compound allopurinol dispersible tablet. The tablet is prepared by the following steps of: respectively sieving 100g of allopurinol, 20g of benzbromarone, 22g of sodium starch glycolate and 4g of superfine silica gel powder by a sieve of 80 meshes; putting 104g of microcrystalline cellulose in an oven at the temperature of 80 DEG C, baking for 2 hours, and sieving by the sieve of 80 meshes for later use; taking the prescription amounts of allopurinol and benzbromarone, and uniformly mixing by an equivalent amount increasing method; taking the prescription amounts of sodium starch glycolate and microcrystalline cellulose and half of the prescription amount of superfine silica gel powder, adding into the mixture of allopurinol and benzbromarone by the equivalent amount increasing method, and uniformly mixing; taking the mixture, using water as an adhesive to make damp mass, sieving by a sieve of 24 meshes to granulate, drying for 3 hours at the temperature of 60-70 DEG C, and arranging particles by a sieve of 22 meshes; uniformly mixing the left half of the prescription amount of superfine silica gel powder with the particles and tabletting, and the weight of each tablet is 0.25g.
Owner:HUBEI GUANGREN PHARMACEUTICAL CO LTD
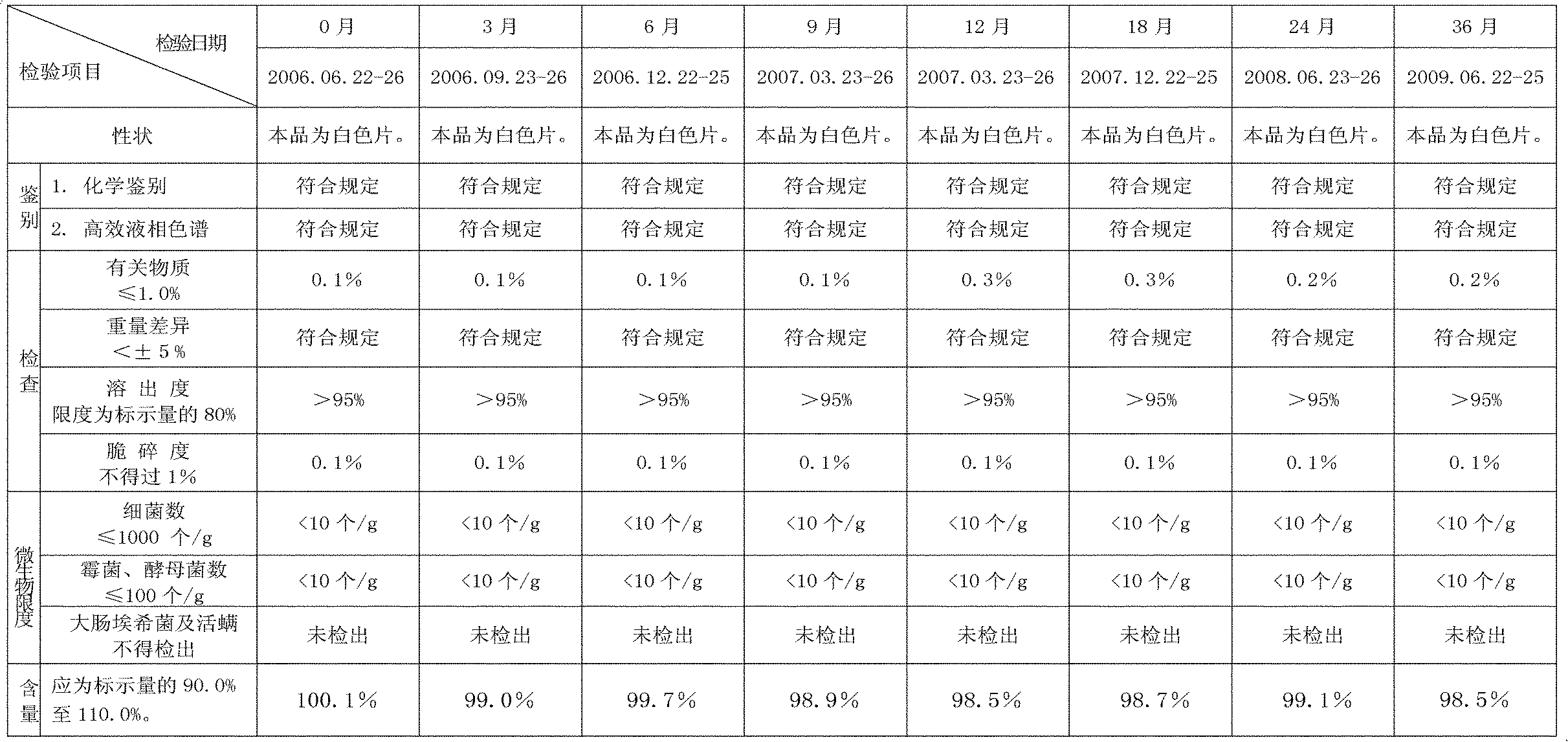
Capecitabine rapidly disintegrating tablets
InactiveUS20110027374A1Superior processing propertySuperior end-product performancePowder deliveryOrganic active ingredientsCrospovidonesLow-substituted hydroxypropylcellulose
There is provided a film coated pharmaceutical composition comprising 5′-deoxy-5-fluoro-N-[(pentyloxy)-carbonyl]-cytidine (capecitabine) and at least one disintegrant selected from the group comprising of crospovidone (particle size <15-400μ), croscarmellose sodium, sodium starch glycolate, low-substituted hydroxypropylcellulose, Ludiflash® or any combination of these, together with other pharmaceutically acceptable excipients to form a rapidly disintegrating tablet.
Owner:BACHYNSKY MARIA OKSANA +3
Lamivudine tablet composition and preparation method thereof
ActiveCN102327249BDisintegrates quicklyGood lookingOrganic active ingredientsPharmaceutical delivery mechanismSoft materialsSodium Starch Glycolate
The invention relates to a lamivudine tablet composition. The core of the lamivudine tablet composition is prepared from the following components: 80-120 parts of lamivudine, 50-90 parts of microcrystalline cellulose, 20-30 parts of starch, 14-22 parts of sodium starch glycolate, 1-4 parts of magnesium stearate and 70-130ml of 50% ethanol solution of 5% povidone K30. The preparation method of thelamivudine tablet composition comprises the following steps: screening; weighing povidone K30, and preparing into a 50% ethanol solution of 5% povidone K30 for later use; weighting lamivudine, starchand part of microcrystalline cellulose, and uniformly mixing to obtain mixed powder; adding the mixed powder obtained in the previous step into the 50% ethanol solution of 5% povidone K30 to prepare a soft material, pelletizing, drying, and granulating to obtain particles; adding sodium starch glycolate, magnesium stearate and the rest of microcrystalline cellulose into the particles obtained in the previous step, and totally mixing; after determining that the lamivudine content is qualified, calculating the required weight of each tablet; tabletting, and coating with coating layers; and carrying out full inspection, and packaging.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Cefetamet pivoxil hydrochloride dispersible tablet and preparation method thereof
InactiveCN102860990APromote dissolutionGood dispersionAntibacterial agentsOrganic active ingredientsDissolutionSodium Starch Glycolate
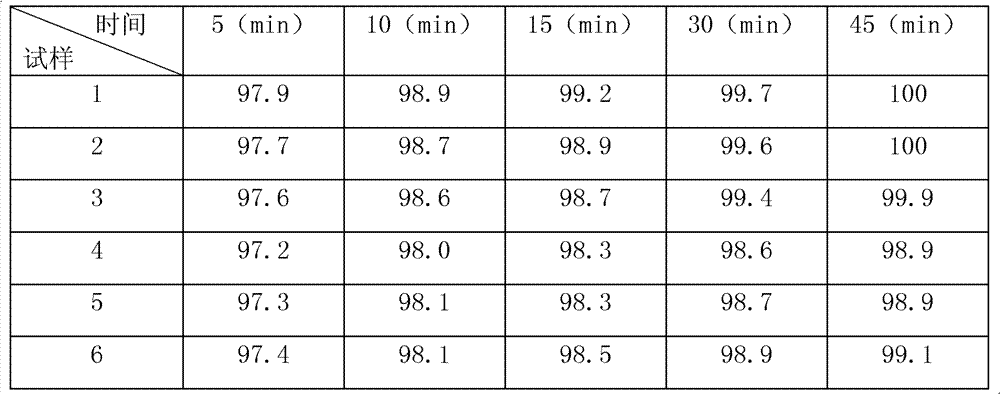
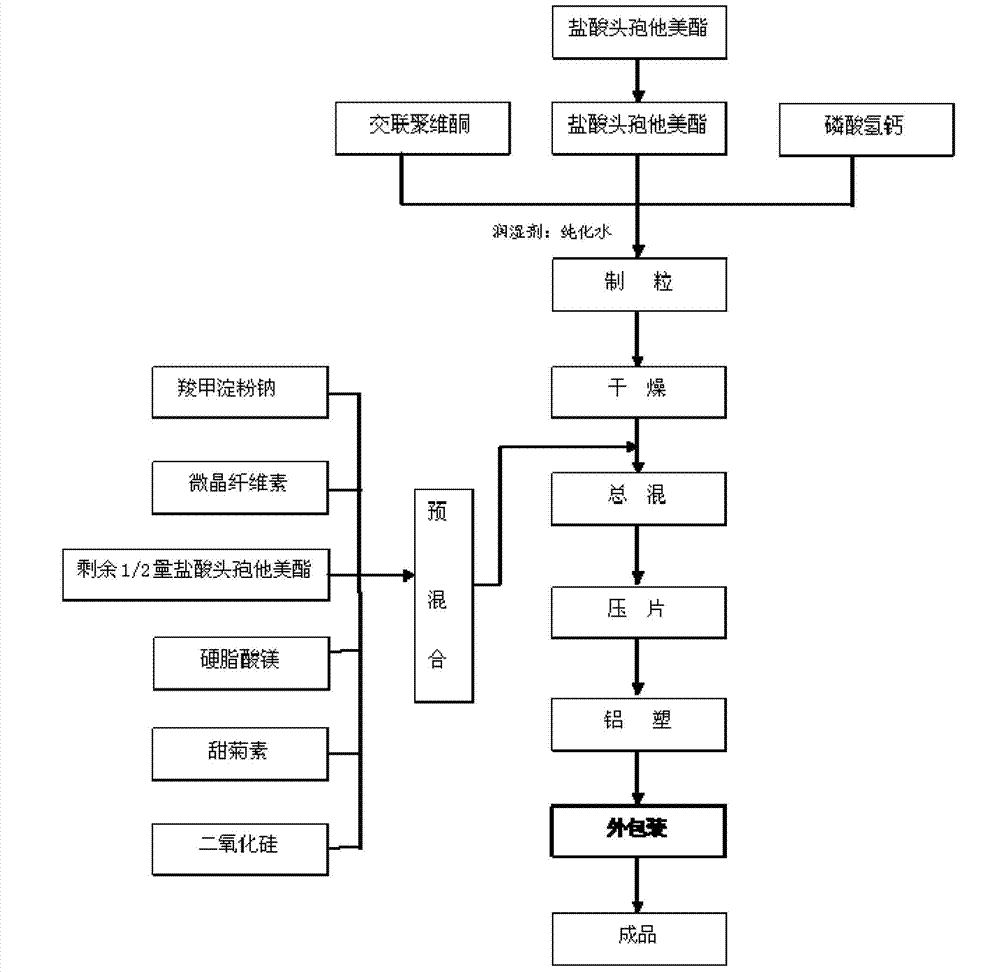
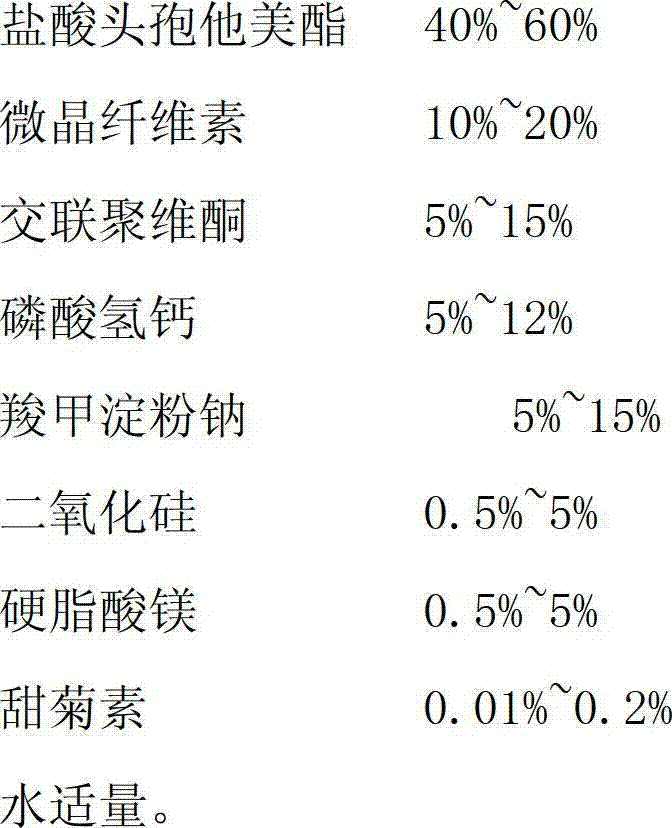
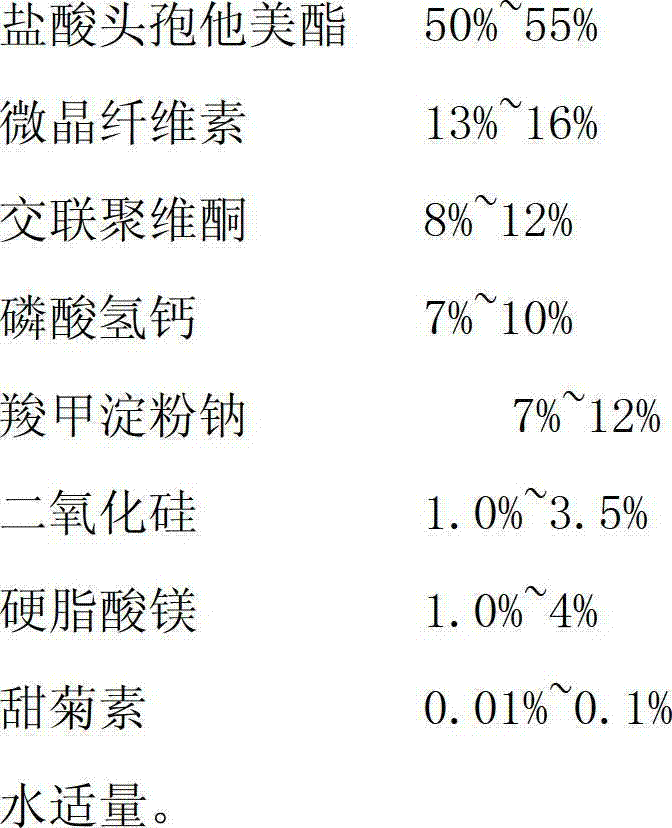
The invention relates to the medical field, in particular to a cefetamet pivoxil hydrochloride dispersible tablet and a preparation method thereof. The cefetamet pivoxil hydrochloride dispersible tablet is prepared by the following components, by weight: 40-60% of cefetamet pivoxil hydrochloride, 10-20% of microcrystalline cellulose, 5-15% of polyvinylpolypyrrolidone, 5-12% of calcium hydrophosphate, 5-15% of sodium starch glycolate, 0.5-5% of silicon dioxide, 0.5-5% of magnesium stearate, 0.01-0.2% of steviosin and a proper amount of water. Compared with the ordinary tablets and capsules, the cefetamet pivoxil hydrochloride dispersible tablet has the advantages of being good in dispersing state, short in disintegration time, fast in medicine dissolution and absorption, high in bioavailability, few in bar reaction, convenient to take and the like.
Owner:ZHEJIANG KAIRUN PHARMA
Extended release excipient and its use
A controlled release excipient composition suitable in formulation of a slow or extended release tablet, contains a synergistic mixture of substantially uncross-linked carboxymethyl starch, or sodium starch glycolate (SSG), and a hydrophilic, non-ionic cellulose ether, preferably hydroxypropylmethylcellulose. Whether or not a SSG in the mixture is sufficiently uncross-linked in the context of the invention can be determined by sedimentation: 0.25 g of the formulation in 100 ml deionized water after 24 hours at 25° C., if subjected to centrifugation at 6080 G at 25° C. for 15 minutes, should exhibit a sedimentation volume of more than 60 ml.
Owner:FRIESLANDCAMPINA NEDERLAND BV
Four-colored granular Keteling capsules and preparation method thereof
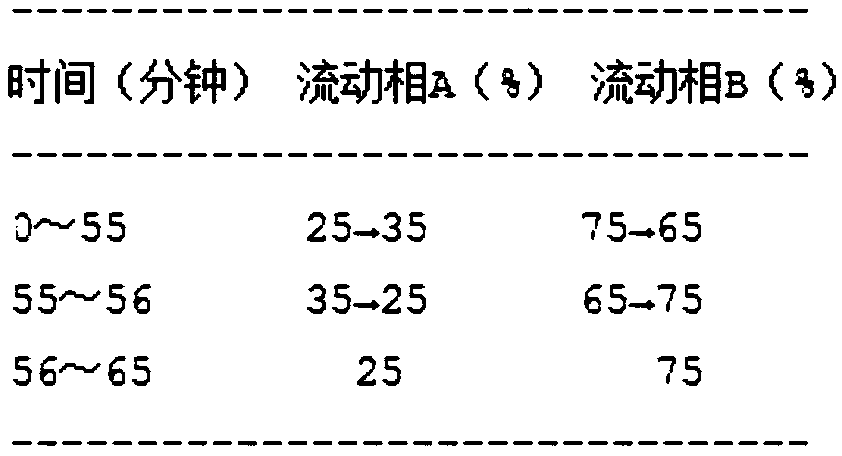
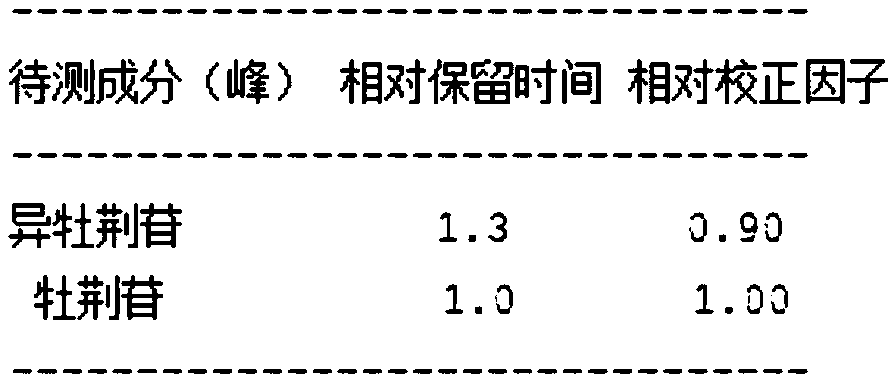
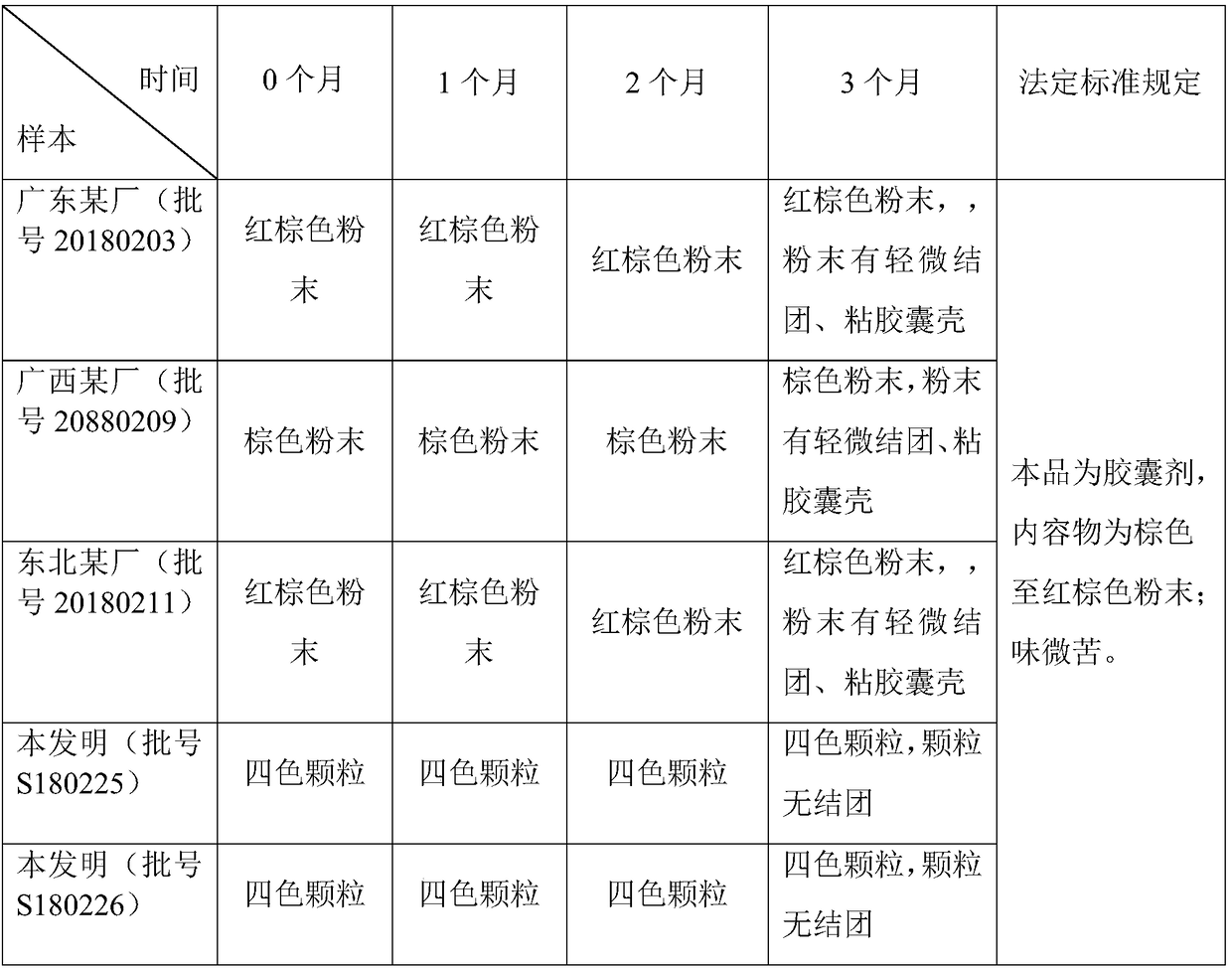
InactiveCN109125400AImprove stabilityShort disintegration timeOrganic active ingredientsInorganic non-active ingredientsThin layerSodium Starch Glycolate
The invention discloses four-colored granular Keteling capsules. The four-colored granular Keteling capsules comprises a component A, a component B, a component C and a component D, wherein the component A is a ficus microcarpa dry extract; the component B comprises chlorpheniramine maleate and a proper amount of starch; the component C is starch; the component D is sodium starch glycolate; the component A, the component B, the component C and the component D are separately coated and granulated, and are then mixed to obtain the capsules. The invention further discloses a preparation method ofthe four-colored granular Keteling capsules. Aspects of indexes of correlation and the like such as the character, water, disintegration time, thin-layer identification, the content of the ficus microcarpa dry extract and the content of the chlorpheniramine maleate of the four-colored granular Keteling capsules conform to the rule of the pharmacopoeia criterion, wherein the stability of the content of the ficus microcarpa dry extract and the stability of the content of the chlorpheniramine maleate are remarkably higher than the stability of conventional sold products. Due to stable content, short disintegration time, rapid dissolving and rapid absorption, the efficacy is good.
Owner:广东罗浮山药业有限公司
Liver aid troche and preparation method thereof
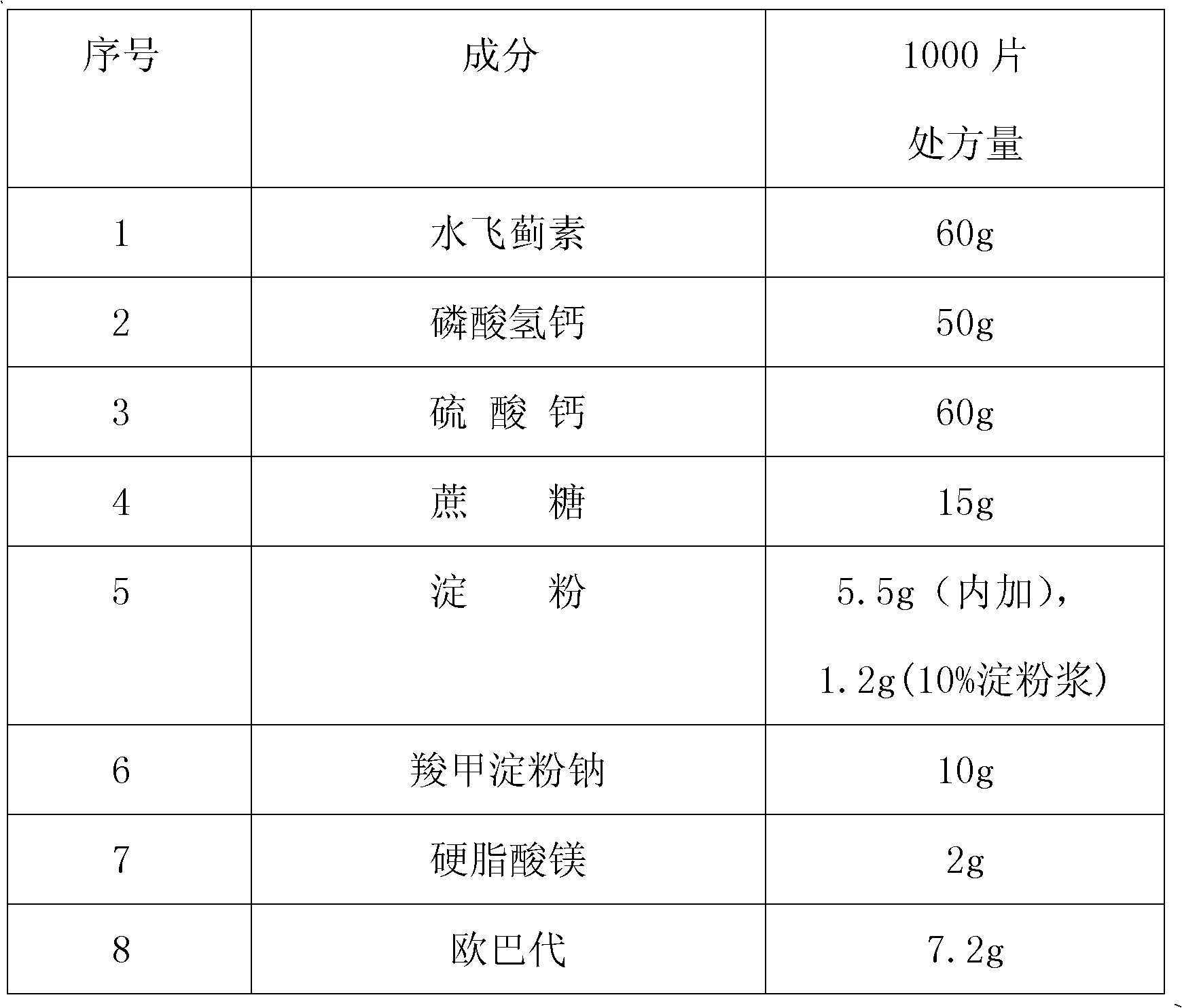
InactiveCN102488666AReduce generationIncrease productivityOrganic active ingredientsDigestive systemSucroseWaste product
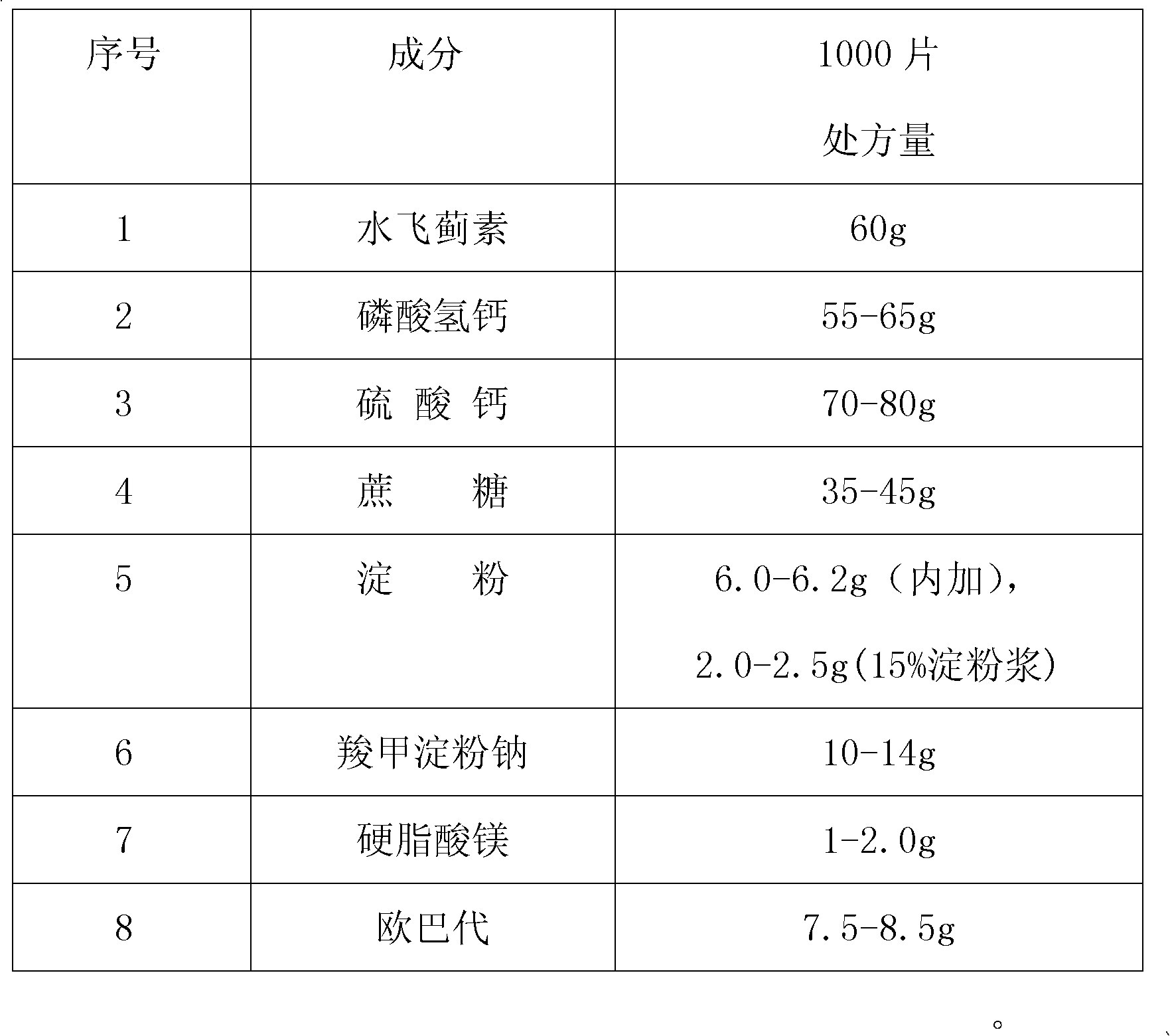
The invention provides a liver aid troche, which is characterized by comprising the following components in weight ratio: in each 1000 troches: 60 g of silymarin, 55-65 g of calcium hydrophosphate, 70-80 g of calcium sulphate, 35-45 g of cane sugar, 6.0-6.2 g of starch added with 2.0-2.5 g of starch slurry which is 15%, 10-14 g of sodium starch glycolate, 1-2.0 g of magnesium stearate, and 7.5-8.5 g of Opadry. The liver aid troche disclosed by the invention is formed by adopting a novel preparation method and screening a more appropriate formula; therefore, the weight difference in the tabletting process meets the requirement, and mass production is smooth to carry out; simultaneously, the phenomena of being uneven in troche surface, pocked and broken after troches are coated can be solved; the rejection rate is reduced; the production efficiency is increased; and the method disclosed by the invention is simple, free from addition of new cost, easy for scale production and higher in application value.
Owner:SHANGHAI ZHAOHUI PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com