Letrozole tablet and preparation method thereof
A letrozole tablet and auxiliary material technology, applied in the field of letrozole tablet and its preparation, can solve the problems of poor dissolution rate, poor water solubility, dust pollution, etc., and achieve the effect of increased dissolution rate and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
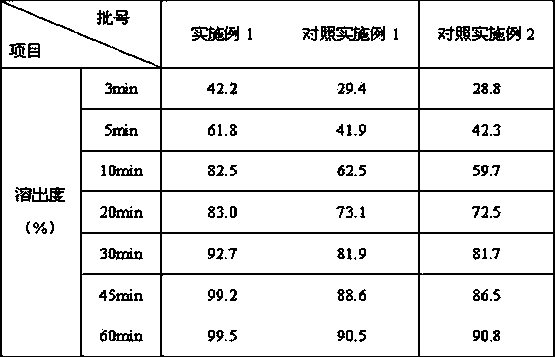
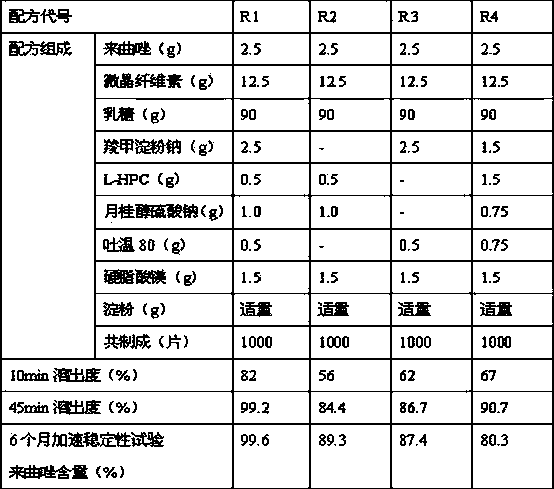
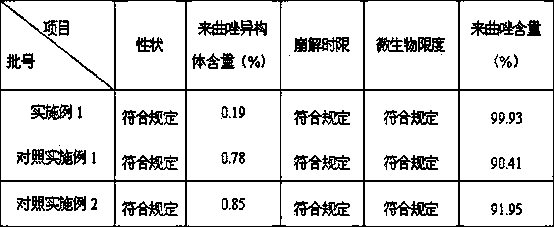
[0018] Embodiment 1: the preparation of letrozole tablet
[0019] Letrozole prescription:
[0020] Letrozole 2.5g
[0021] Microcrystalline Cellulose 12.5g
[0022] Lactose 90g
[0023] Sodium starch glycolate 2.5g
[0024] L-HPC 0.5g
[0025] Sodium Lauryl Sulfate 1.0g
[0026] Tween 80 0.5g
[0027] Magnesium Stearate 1.5g
[0028] Appropriate amount of starch
[0029] A total of 1000 pieces were made
[0030] Preparation:
[0031] Adhesive preparation: take starch, add appropriate amount of water, stir well, add water and boil to obtain starch slurry with a concentration of about 2.5%, add Tween-80 and mix well;
[0032] Crush and sieve: take the letrozole raw material, ultrafinely pulverize, and pass the auxiliary materials microcrystalline cellulose, lactose, sodium starch glycolate, L-HPC, and sodium lauryl sulfate through an 80-mesh sieve;
[0033] Mixing and granulation: Take the pulverized letrozole and mix it with lactose in equal increments, pass th...
Embodiment 1
[0036] Comparative example 1: the preparation of letrozole tablet
[0037] Letrozole prescription:
[0038] Letrozole 2.5g
[0039] Polyvinylpyrrolidone K30 4.375g
[0040] Tween 80 1.875g
[0041] Mannitol 3.75g
[0042] Lactose 62.5g
[0043] Microcrystalline Cellulose 56.25g
[0044] Cross-linked polyvinylpolypyrrolidone 5g
[0045] Magnesium stearate 0.625g, made into 1000 tablets.
[0046] Preparation:
[0047] Add polyvinylpyrrolidone K30 (PVP K30), polysorbate 80, and mannitol of prescription quantity into 400ml water, stir to form a uniform solution, add letrozole in the solution, and ultrasonically disperse it for 5 minutes to obtain a suspension; Mix the prescribed amount of lactose, microcrystalline cellulose, and cross-linked polyvinylpolypyrrolidone (PVPP) evenly, spray the above suspension into the mixed auxiliary materials through a spray gun with a peristalt...
Embodiment 2
[0048] Comparative example 2: the preparation of letrozole tablet
[0049] Letrozole 2.5g
[0050] Sorbitol 12 g
[0051] Dextrin 57.4g
[0052] Aspartame 1.7 g
[0053] Cross-linked polyvinylpyrrolidone 7.5 g
[0054] Polysorbate 80 0.1 g
[0055]Hypromellose 0.6 g
[0056] Orange essence 1.0 g, made into 1000 pieces.
[0057] Preparation:
[0058] (1) Letrozole is pulverized through a 170 mesh sieve for subsequent use;
[0059] (2) All auxiliary materials are passed through an 80-mesh sieve for subsequent use;
[0060] (3) Sorbitol, dextrin, aspartame, and cross-linked polyvinylpyrrolidone are weighed and mixed uniformly to obtain material I. Letrozole is taken in an equal amount and added to material I in equal amounts, mixed uniformly to obtain material II;
[0061] (4) Add polysorbate 80 to 2% hypromellose aqueous solution to make a binder, add an appropri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com