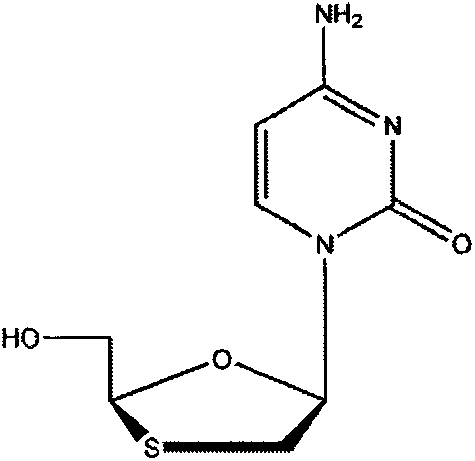
Lamivudine tablet composition and preparation method thereof
A lamivudine tablet and a lamivudine technology, which are applied in the field of medicine, can solve problems such as unsatisfactory disintegration effect, and achieve the effects of good taste, not easy to absorb moisture, and delicate appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
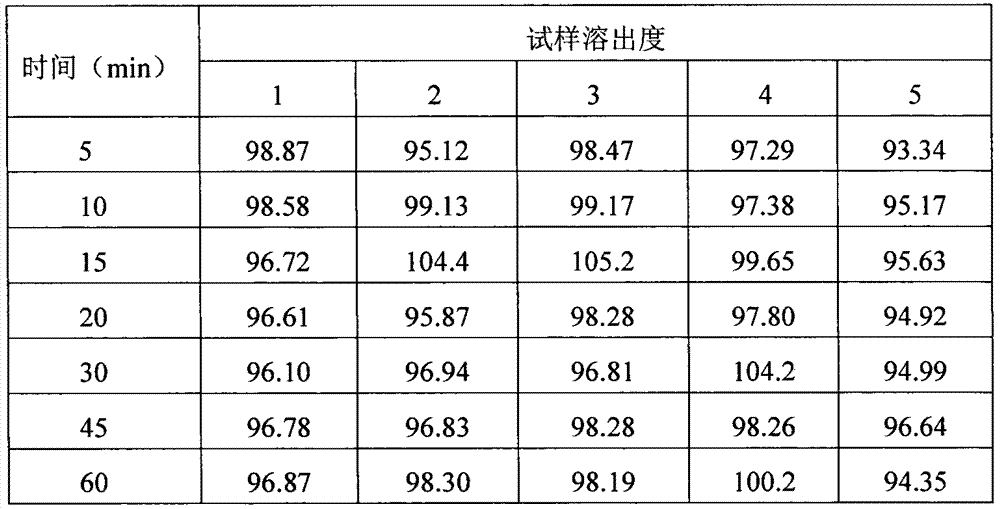
[0040] Get the raw and auxiliary materials lamivudine, microcrystalline cellulose, sodium starch glycolate, silicon dioxide, and magnesium stearate, and pass through an 80-mesh sieve. Take by weighing 100g of lamivudine, 130g of microcrystalline cellulose, 10g of sodium starch glycolate, 2g of silicon dioxide, and 2g of magnesium stearate, mix uniformly, measure the content of mixed intermediates, and calculate the tablet weight after passing the test. Direct compression. Get lamivudine tablet core, put in coating pan, carry out coating with coating solution (70% ethanol solution of hypromellose, polysorbate 80, macrogol 4000, titanium dioxide), until weight gain 2%, the coated tablets are dried and solidified to obtain finished lamivudine tablets, which are fully inspected and packaged.
Embodiment 2
[0042] Get the raw and auxiliary materials lamivudine, microcrystalline cellulose, sodium starch glycolate, silicon dioxide, and magnesium stearate, and pass through a 90-mesh sieve. Take by weighing 100g of lamivudine, 130g of microcrystalline cellulose, 10g of sodium starch glycolate, 2g of silicon dioxide, and 2g of magnesium stearate, mix uniformly, measure the content of mixed intermediates, and calculate the tablet weight after passing the test. Direct compression. Get lamivudine tablet core, put in coating pan, carry out coating with coating solution (70% ethanol solution of hypromellose, polysorbate 80, macrogol 4000, titanium dioxide), until weight gain 3%, the coated tablets are dried and solidified to obtain finished lamivudine tablets, which are fully inspected and packaged.
Embodiment 3
[0044] Get the raw and auxiliary materials lamivudine, microcrystalline cellulose, sodium starch glycolate, silicon dioxide, and magnesium stearate, and pass through a 100-mesh sieve. Take by weighing 100g of lamivudine, 130g of microcrystalline cellulose, 10g of sodium starch glycolate, 2g of silicon dioxide, and 2g of magnesium stearate, mix uniformly, measure the content of mixed intermediates, and calculate the tablet weight after passing the test. Direct compression. Get lamivudine tablet core, put in coating pan, carry out coating with coating solution (70% ethanol solution of hypromellose, polysorbate 80, macrogol 4000, titanium dioxide), until weight gain 4%, after the coated tablet is dried and solidified, the finished product of lamivudine tablet is obtained, which is obtained after full inspection and packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com