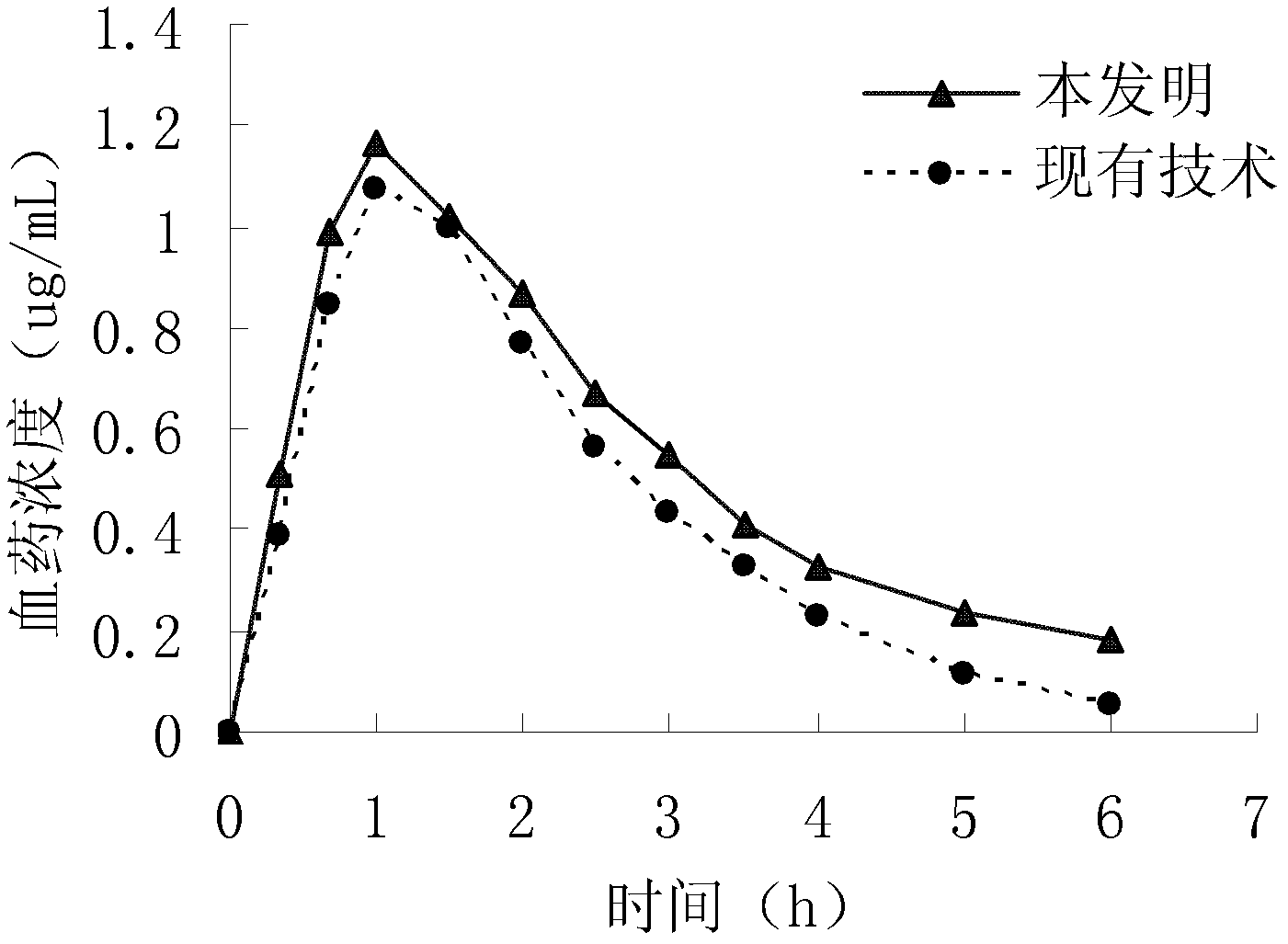
Cefteram pivoxil crystal and preparation method thereof and composition tablets containing crystal
A technology for cefdipram pivoxil and a composition, which is applied in the field of pharmaceutical preparations, and can solve the problems of easy decomposition, affecting drug dissolution effect and bioavailability, insoluble in water, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102][embodiment 1] the preparation of cefditoren neopentyl ester crystal
[0103] 1) cefditoren neopentyl powder is dissolved in an acetone solvent to form a cefditoren neopentyl solution as a dispersed phase;
[0104] 2) dissolving polyvinylpyrrolidone in water to form an aqueous solution of polyvinylpyrrolidone as a dispersion medium;
[0105] 3) Under the ultrasonic field, use a micro-injector to add the dispersed phase solution into the dispersion medium, continue ultrasonication, and centrifuge to separate the cefditoren neopentyl superpowder, remove the supernatant and add water to wash and centrifuge to remove the cephalosporin The polyvinylpyrrolidone adsorbed on the surface of telen neopentyl ester is vacuum-dried to obtain cefditoren neopentyl ester crystals.
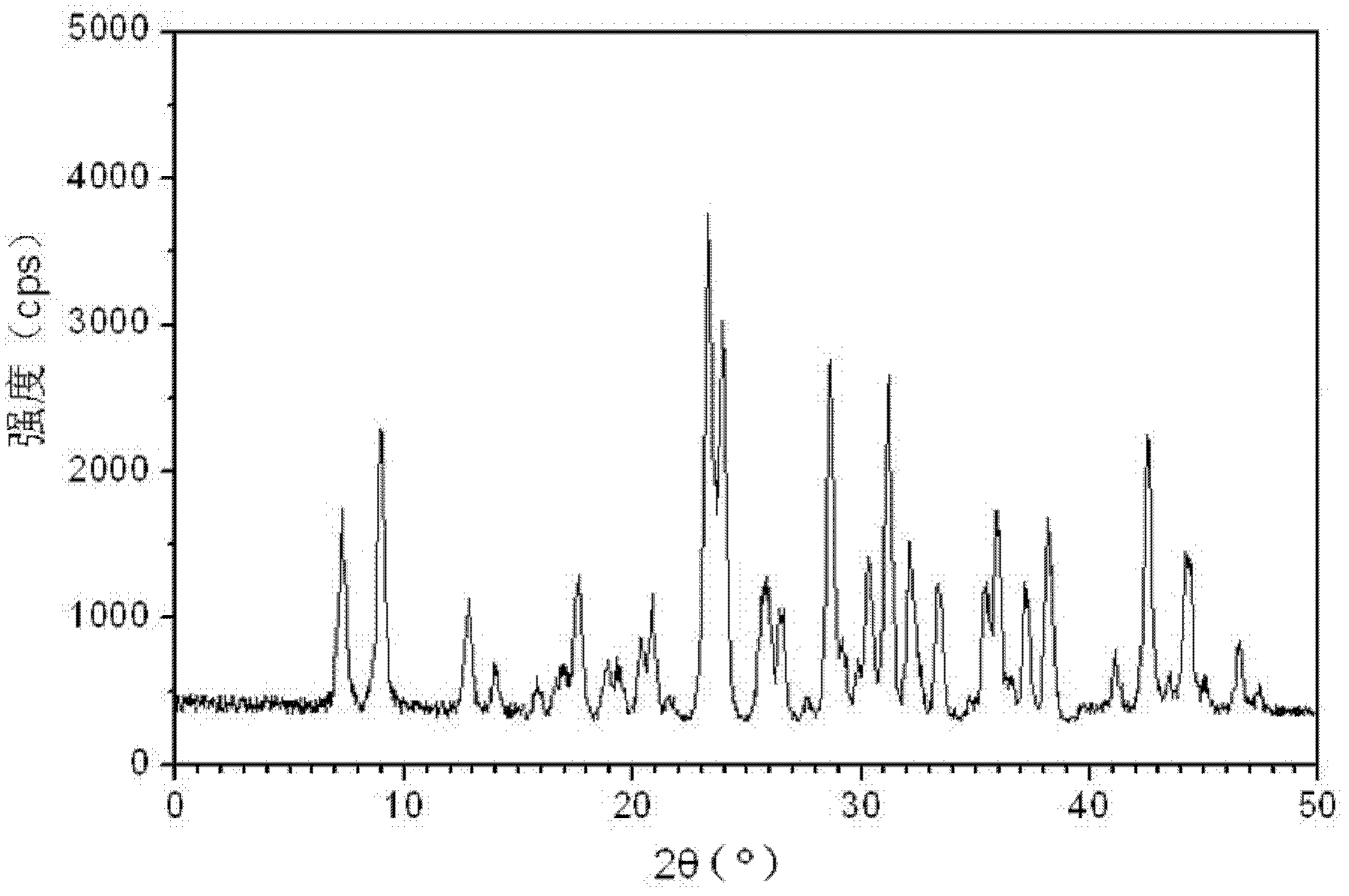
[0106] The particle diameter of the obtained cefditoren neopentyl crystal is 1 μm, and it is measured by powder X-ray diffractometry, and the X-ray powder diffraction spectrum represented by 2θ ± 0.2 ° diff...
Embodiment 2
[0107] [embodiment 2] the preparation of cefditoren neopentyl ester crystal
[0108] 1) cefditoren neopentyl powder is dissolved in an acetone solvent to form a cefditoren neopentyl solution as a dispersed phase;
[0109] 2) Dissolve polyvinylpyrrolidone in water to make 0.05mol L -1 Polyvinylpyrrolidone aqueous solution is used as dispersion medium;
[0110] 3) Under an ultrasonic field with a power of 0.1KW, add the dispersed phase solution into the dispersion medium with a micro-injector, continue ultrasonication for 1 minute, and -1 Cefditoren neopentyl superpowder was separated by high-speed centrifugation under certain conditions, the supernatant was removed and water was added to wash and centrifuge twice to remove the polyvinylpyrrolidone adsorbed on the surface of cefditoren neopentyl, and vacuum-dried for 8 hours to obtain cephalosporin Tren pivalate crystals.
[0111] The obtained cefditoren neopentyl crystal has a particle size of 5 μm, and the X-ray powder diff...
Embodiment 3
[0112] [embodiment 3] the preparation of cefditoren neopentyl ester crystal
[0113] 1) cefditoren neopentyl powder is dissolved in an acetone solvent to form a cefditoren neopentyl solution as a dispersed phase;
[0114] 2) Dissolve polyvinylpyrrolidone in water to make 0.3mol L -1 Polyvinylpyrrolidone aqueous solution is used as dispersion medium;
[0115] 3) Under an ultrasonic field with a power of 0.3KW, add the dispersed phase solution into the dispersion medium with a micro-injector, continue ultrasonication for 3 minutes, and -1 Cefditoren neopentyl superpowder was separated by high-speed centrifugation under certain conditions, and the supernatant was removed and washed with water and centrifuged for 4 times to remove the polyvinylpyrrolidone adsorbed on the surface of cefditoren neopentyl, and vacuum-dried for 12 hours to obtain cephalosporin Tren pivalate crystals.
[0116] The obtained cefditoren neopentyl crystals had a particle size of 3 μm, and the X-ray powd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com