Glimepiride tablet and preparation method thereof
A glimepiride tablet and tablet compression technology, applied in the field of medicine, can solve the problems of unsatisfactory quality of glimepiride tablets, easy generation of impurities, increase of adverse drug reactions, etc., and achieve better dissolution effect and less impurities , the effect of high economic and social significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1 prepares the glimepiride tablet of the present invention
[0014] Prescription, see Table 1:
[0015] Table 1 Example 1 prescription component list
[0016] Material name Dosage (g) / 1000 tablets Glimepiride (g) 1 Lactose (g) 74 Microcrystalline Cellulose (g) 3.2 Sodium starch glycolate (g) 4.25 Povidone K30(g) 1.7 Magnesium stearate (g) 0.85
[0017] Preparation:
[0018] (1) Add 10 g of crude glimepiride to 500 mL of chloroform, heat to reflux to dissolve, then add 100 mL of tetrahydrofuran and 50 mL of tributylmethyl ether to the solution to form a mixed solvent system, then cool down to 0°C for cooling and crystallization, filter, and diethyl ether Washing and vacuum drying at 80°C gave 9.78g of glimepiride with a yield of 97.8%;
[0019] (2) Weigh the prescription amount of glimepiride obtained in step (1), add it to lactose in a weight ratio of 1:2, and finally add the prescription amount of microc...
Embodiment 2
[0033] Embodiment 2 prepares the glimepiride tablet of the present invention
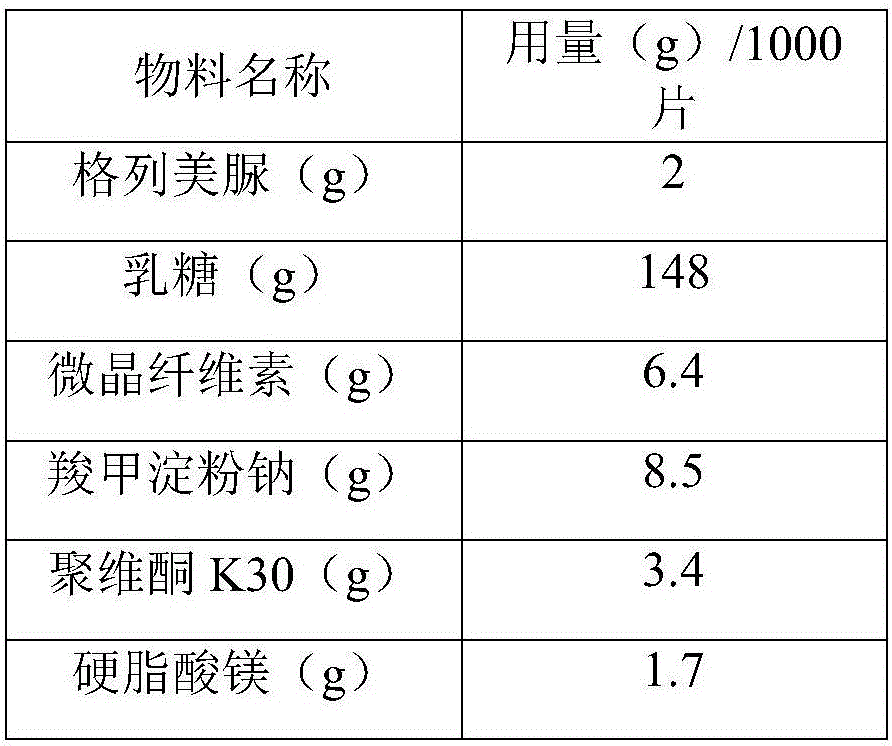
[0034] For prescription, see Table 4.
[0035] Table 4 Example 2 prescription component list
[0036]
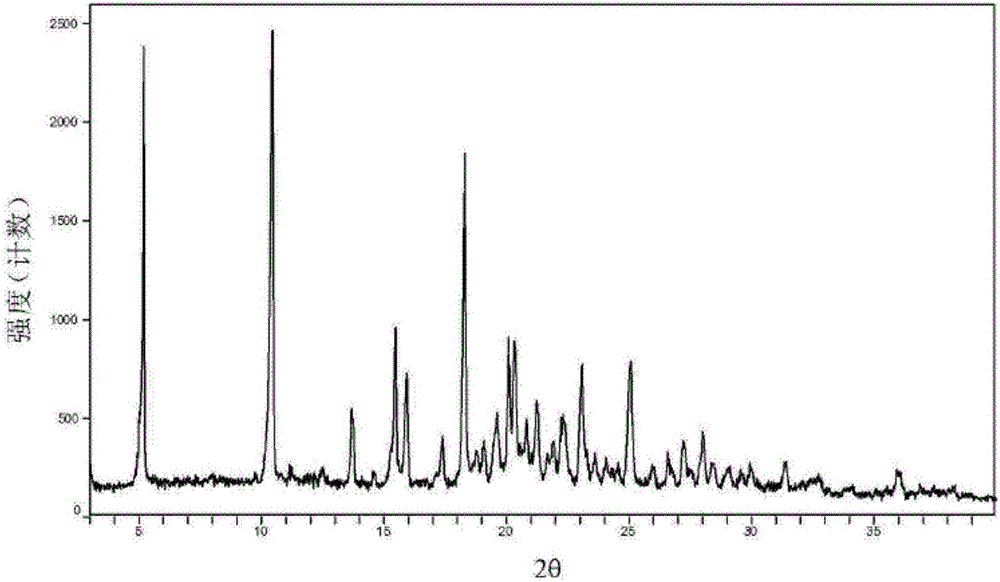
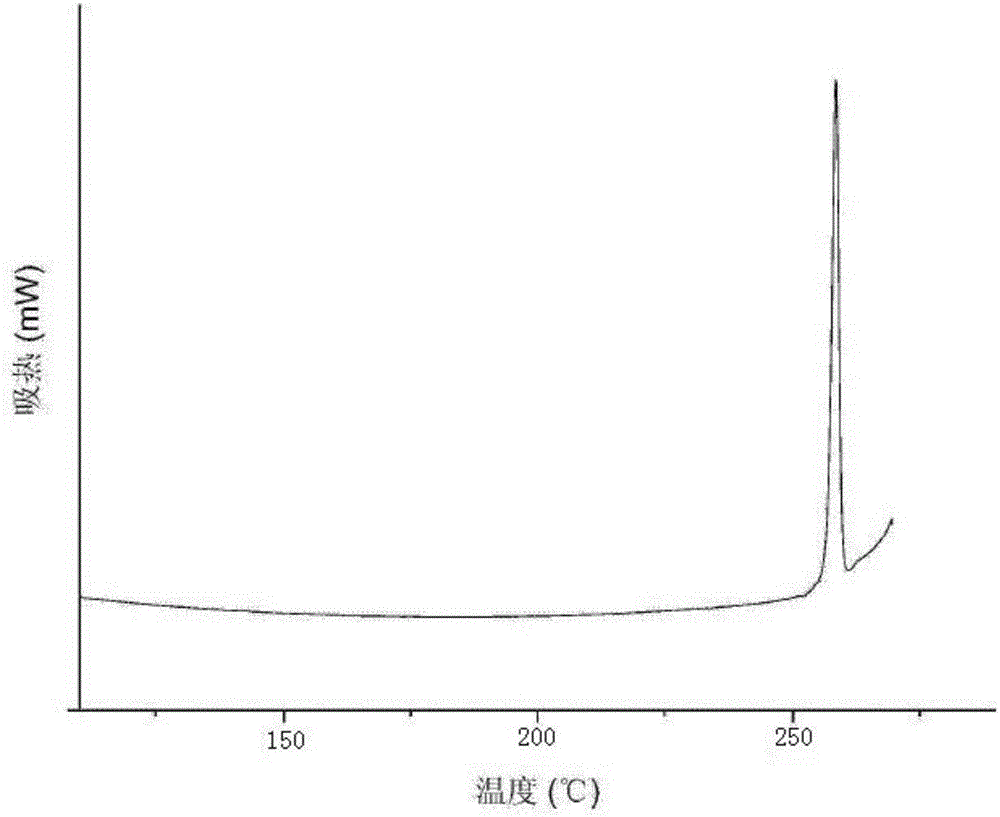
[0037] The preparation method is the same as that in Example 1, and the obtained XRPD spectrum data, differential scanning calorimetry chart data, hygroscopicity test data, and fluidity comparison data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com