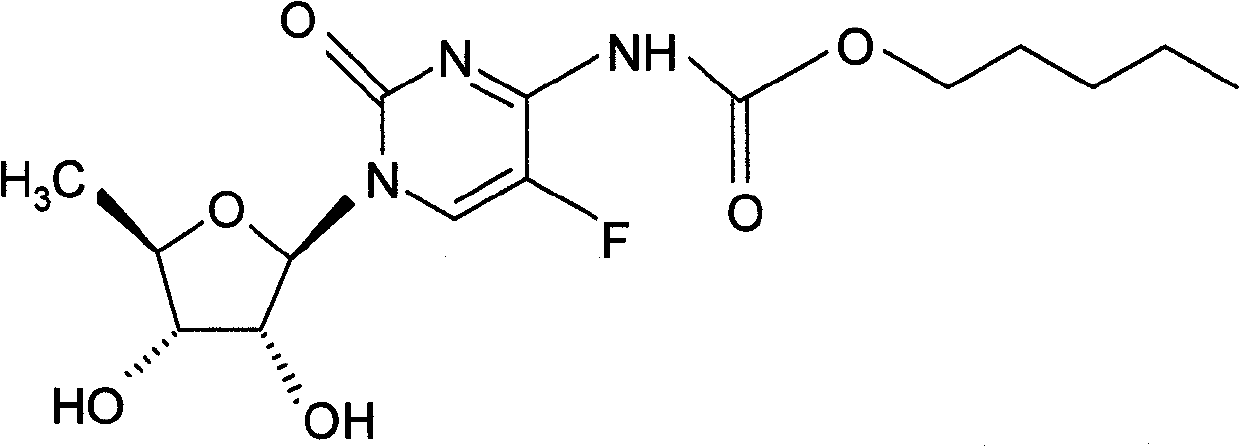
Capecitabine rapidly disintegrating tablets
A technology of capecitabine and disintegrant, which is applied in the direction of pill delivery, coating, organic active ingredients, etc., and can solve problems such as capecitabine’s cohesiveness that cannot be overcome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0028] Preparation composition
[0029]
[0030]
[0031] 1 removed during processing
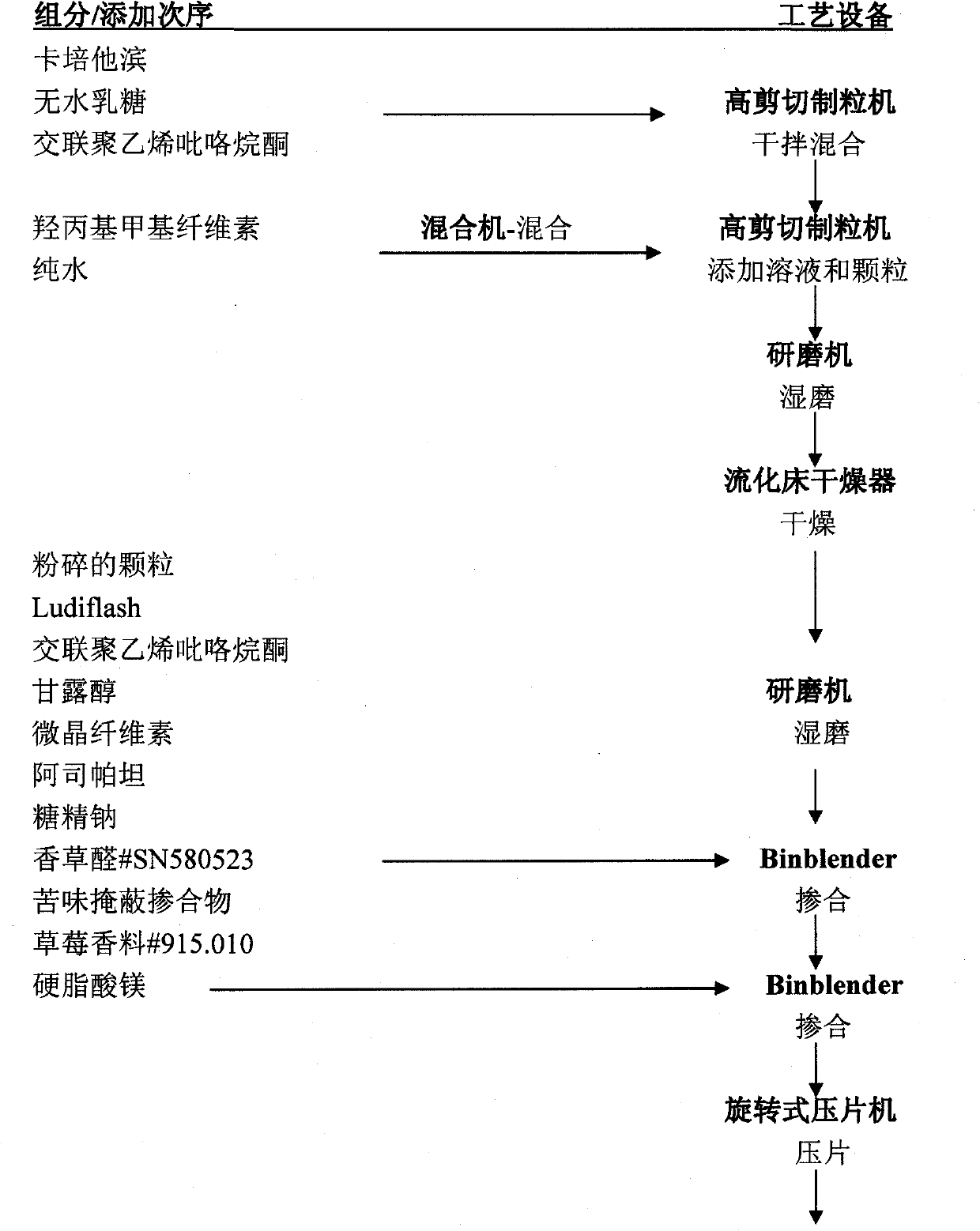
[0032] Manufacturing steps:
[0033] 1. Mix capecitabine with anhydrous lactose and a part of cross-linked polyvinylpyrrolidone
[0034] 2. Dissolve hypromellose in pure water
[0035] 3. Granulate the blend from step 1 with the granulation solution from step 2
[0036] 4. Wet milling of pellets from step 3
[0037] 5. Dry and grind the pellets from step 4
[0038] 6. Combine the pellet from step 5 with Ludiflash The remainder is a blend of crospovidone, mannitol, microcrystalline cellulose, aspartame, sodium saccharin, vanillin, bitter masking blend, and strawberry flavor
[0039] 7. Sift the magnesium stearate, add it to the blend from step 6 and mix
[0040] 8. Compress the tableting mixture from step 7 to nucleate
[0041] 9. Preparation of film coating suspension by dispersing the film coating mixture in purified water
[0042] 10 Film coat the cores from step 8 using ...
Embodiment 7-12
[0044] The following compositions represent preferred formulations in mg / tablet weight where lactose is replaced by mannitol.
[0045]
[0046] 1 removed during processing
[0047] Steps: Similar to the steps of Examples 1-6, except that anhydrous lactose is replaced by mannitol in step 1.
Embodiment 13
[0048] Example 13: Disintegration Characteristics of Dosage Forms
[0049]The following is a comparison of the disintegration times of the rapidly disintegrating tablets of the present invention and commercially available tablets at low and high tablet strengths.
[0050] Disintegration times were obtained using a USP disintegration apparatus without discs and 37°C water. The experimental test methods and resulting observed disintegration times were performed according to the USP disintegration test method (USP 29, General Chapters, Physical Tests, , which is hereby incorporated by reference .
[0051] For the purposes of this test, disintegration does not imply complete dissolution of the unit or even of its active ingredients. Complete disintegration is defined as the state in which, other than insoluble coating or fragments of the capsule shell, any residue of the unit remaining on the sieve of the test device is a soft mass without a distinct film core .
[0052] U...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com