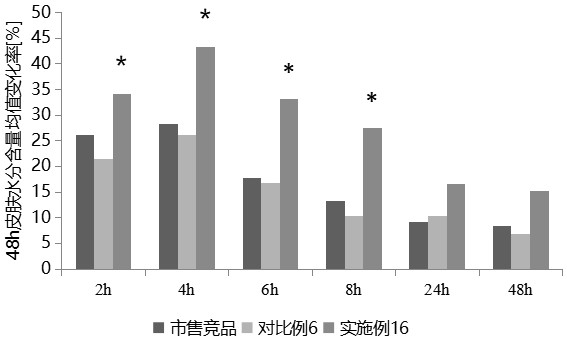
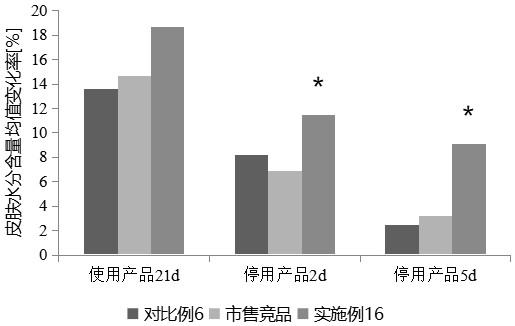
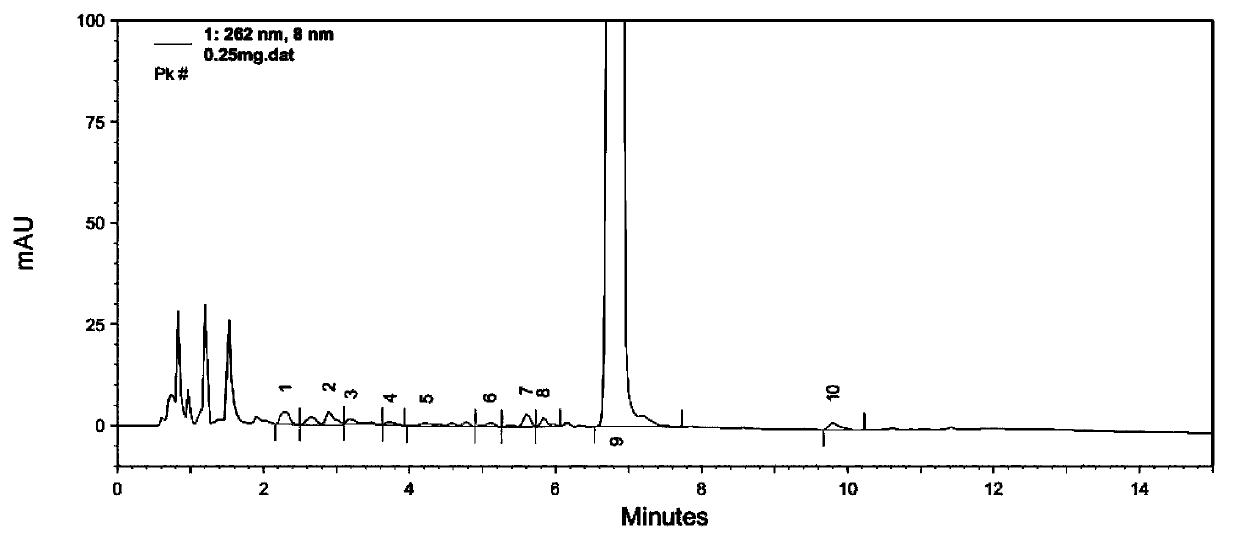
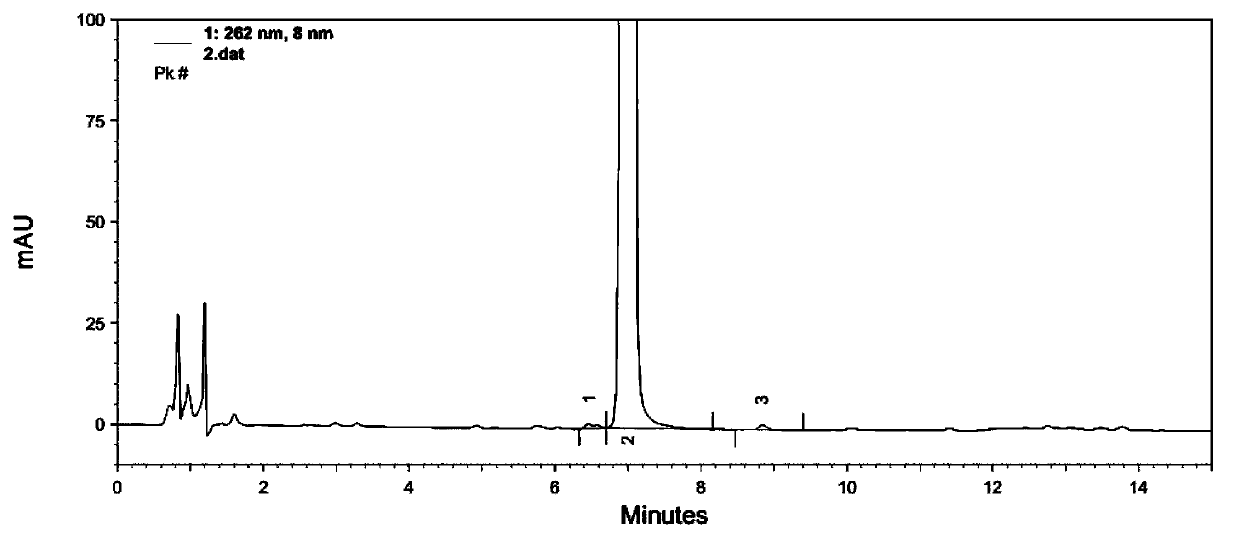
Patents
Literature
46 results about "Pharmasolve" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
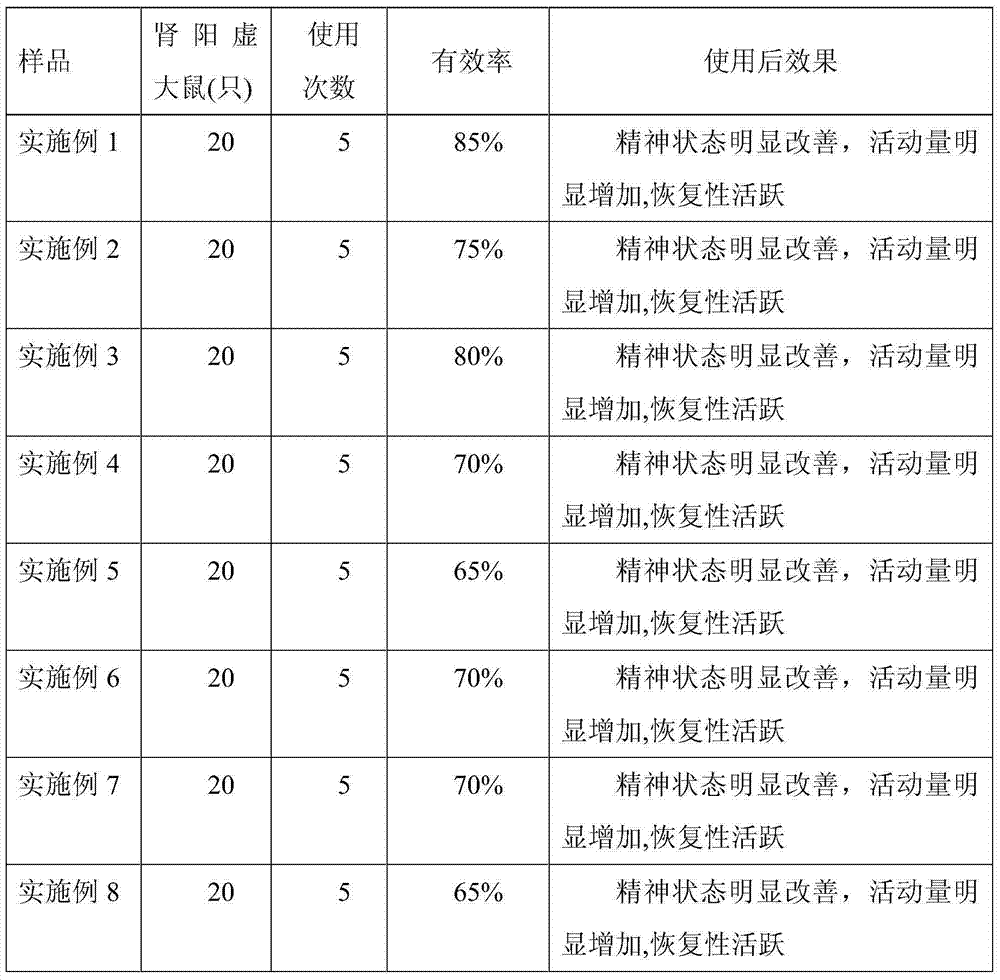
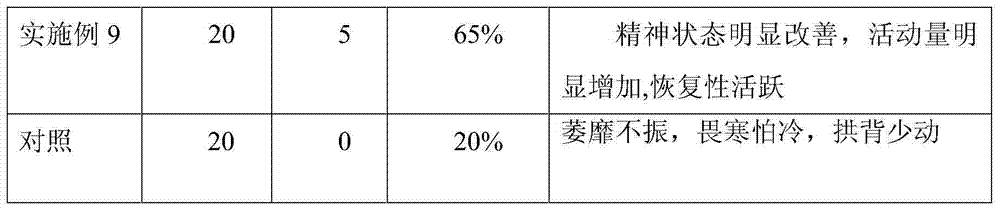
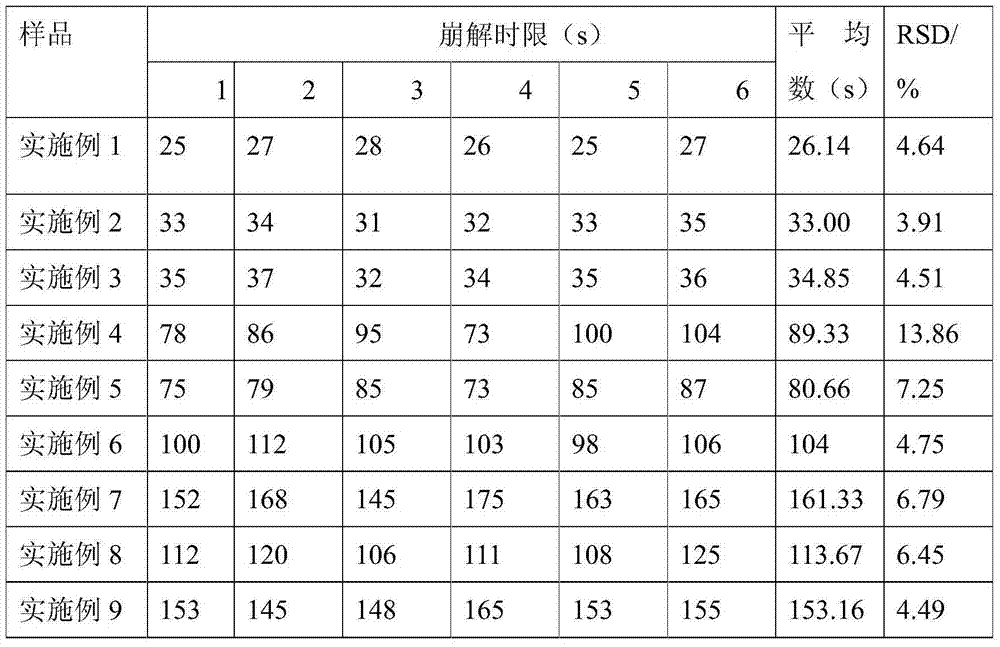
Drug formulation containing a solubilizer for enhancing solubility, absorption, and permeability
InactiveUS20070021325A1Promote absorptionImprove permeabilityOrganic active ingredientsPeptide/protein ingredientsSolubilityBenzoic acid
Solubility, absorption, and permeability of drugs upon oral administration are improved when the drugs are mixed and / or complexed with water-miscible organic solvents. Illustratively, the absorption of a heparin-deoxycholic acid conjugate upon oral administration is increased by mixing and / or complexing this conjugate with dimethyl sulfoxide. Other illustrative water-miscible organic solvents include N-methylpyrrolidone, polyoxyl 35 castor oil, diethylene glycol monoethyl ether, and benzoic acid.
Owner:MEDIPLEX CORP
Dispersible tablet of maca, preparation method and application thereof
ActiveCN103652927APromote dissolutionFast absorptionDispersion deliveryAntinoxious agentsCross-linked polyethyleneChemistry
The invention provides a dispersible tablet of maca. The dispersible tablet is prepared by the following components of: 48%-78% of maca powder, 3%-20% of crospolyvinylpyrrolidone, 0.1%-35% of maltodextrin, 5%-30% of microcrystalline cellulose, 0.03%-1% of sodium lauryl sulfate, 0.1%-3% of aspartame, 0.5%-3% of magnesium stearate and a proper amount of bonding agent. The prepared dispersible tablet of the maca has the advantages that the tablet is high in disintegration and high in dispersing uniformity, has obvious functions for relieving the physical fatigue and improving the sexual function, is good in drug compliance, and is especially applicable to old people and patients with difficulty in swallowing. A preparation method of the dispersible tablet of the maca has the advantages that the process is simple, the operation is controllable, new equipment does not need to be added, and the suitability for industrial and large-scale production is achieved.
Owner:CHONGQING XIXUAN BIOTECH CO LTD
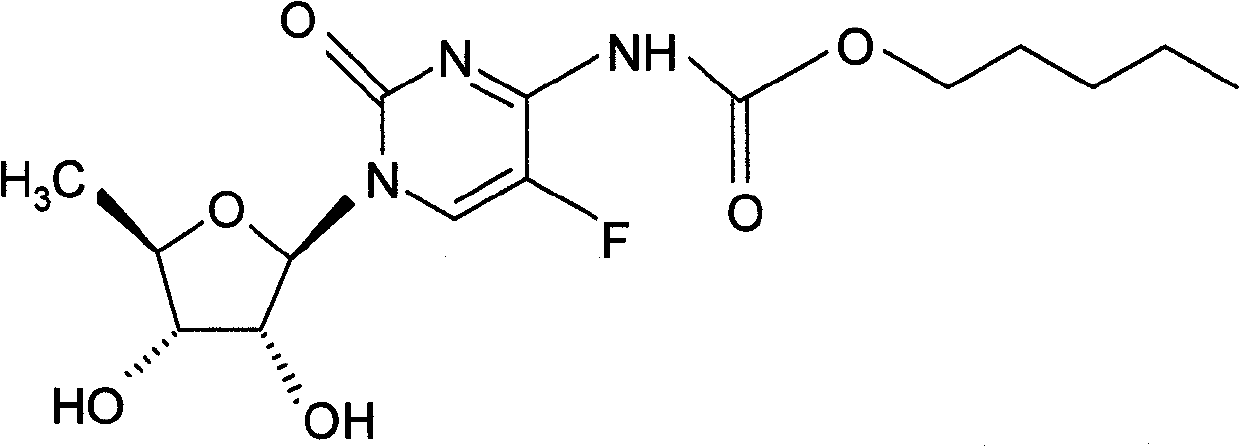
Capecitabine rapidly disintegrating tablets
InactiveCN102369002AOrganic active ingredientsDispersion deliveryCroscarmellose sodiumCarboxymethylcellulose Sodium
There is provided a film coated pharmaceutical composition comprising 5 '-deoxy-5-fluoro-N-[(pentyloxy)-carbonyl]-cytidine (capecitabine) and at least one disintegrant selected from the group comprising of crospovidone (particle size < 15-400 [mu]), croscarmellose sodium, sodium starch glycolate, low-substituted hydroxypropylcellulose, Ludiflash TM or any combination of these, together with other pharmaceutically acceptable excipients to form a rapidly disintegrating tablet.
Owner:F HOFFMANN LA ROCHE & CO AG
Coated tablets
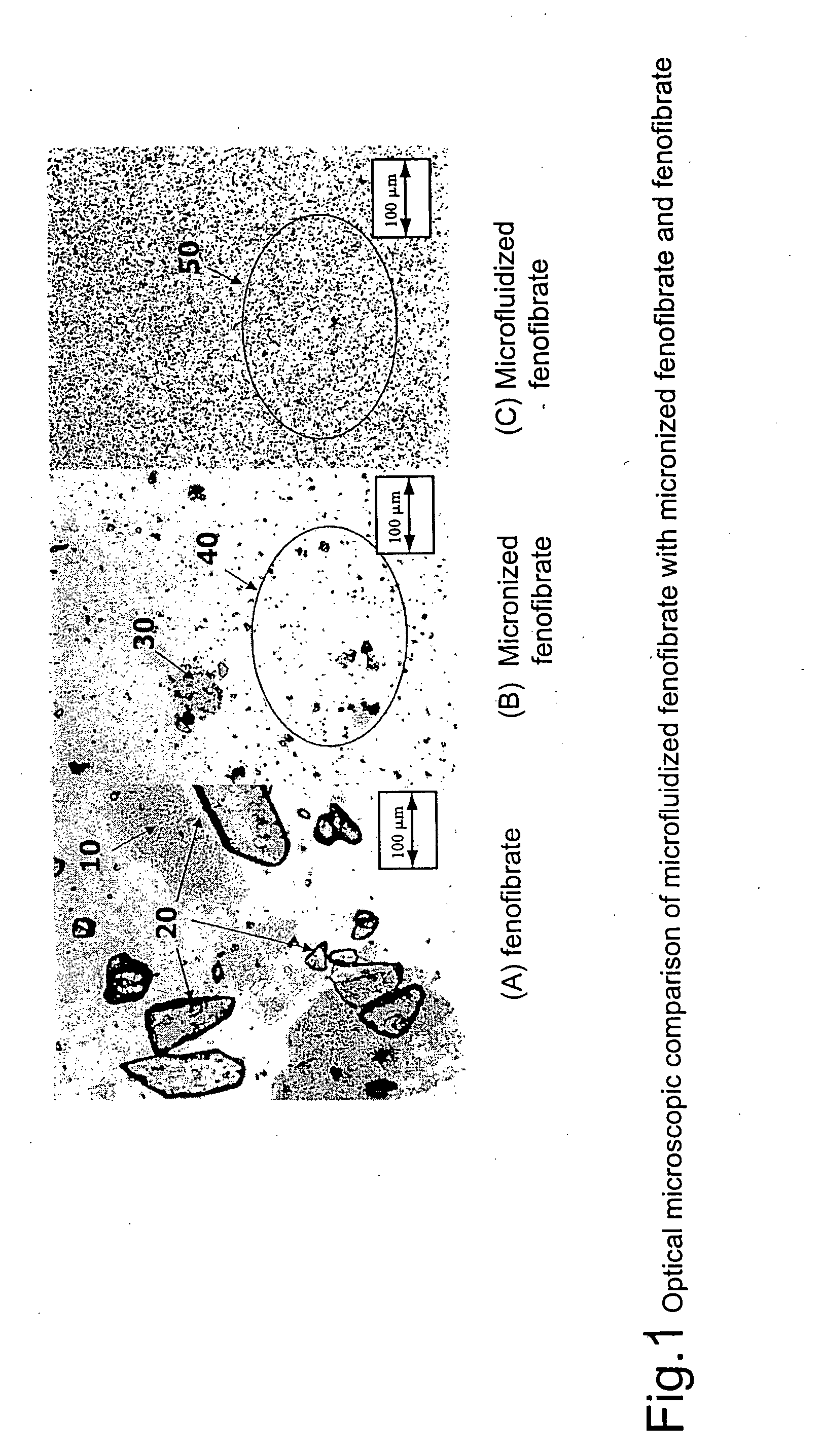
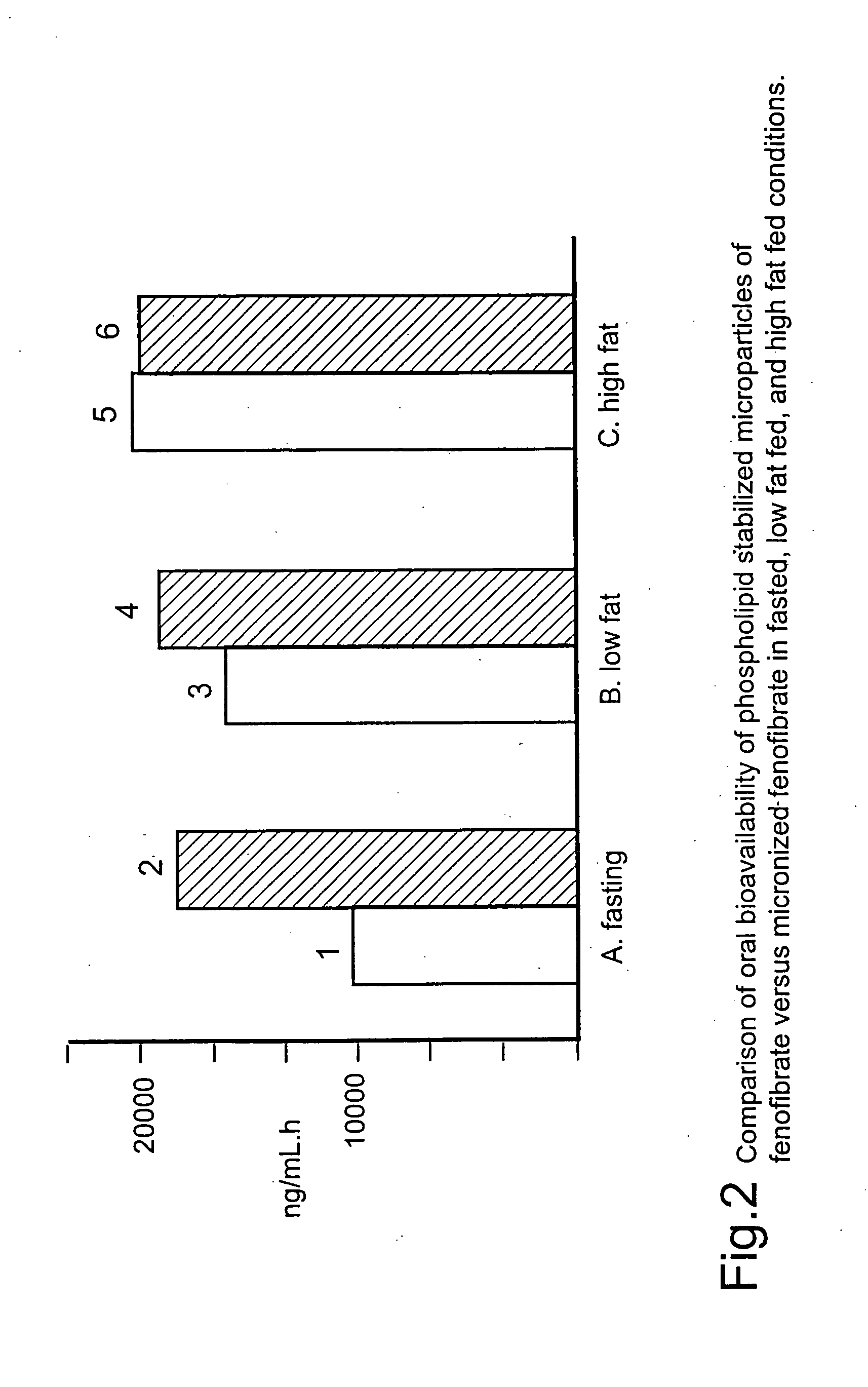
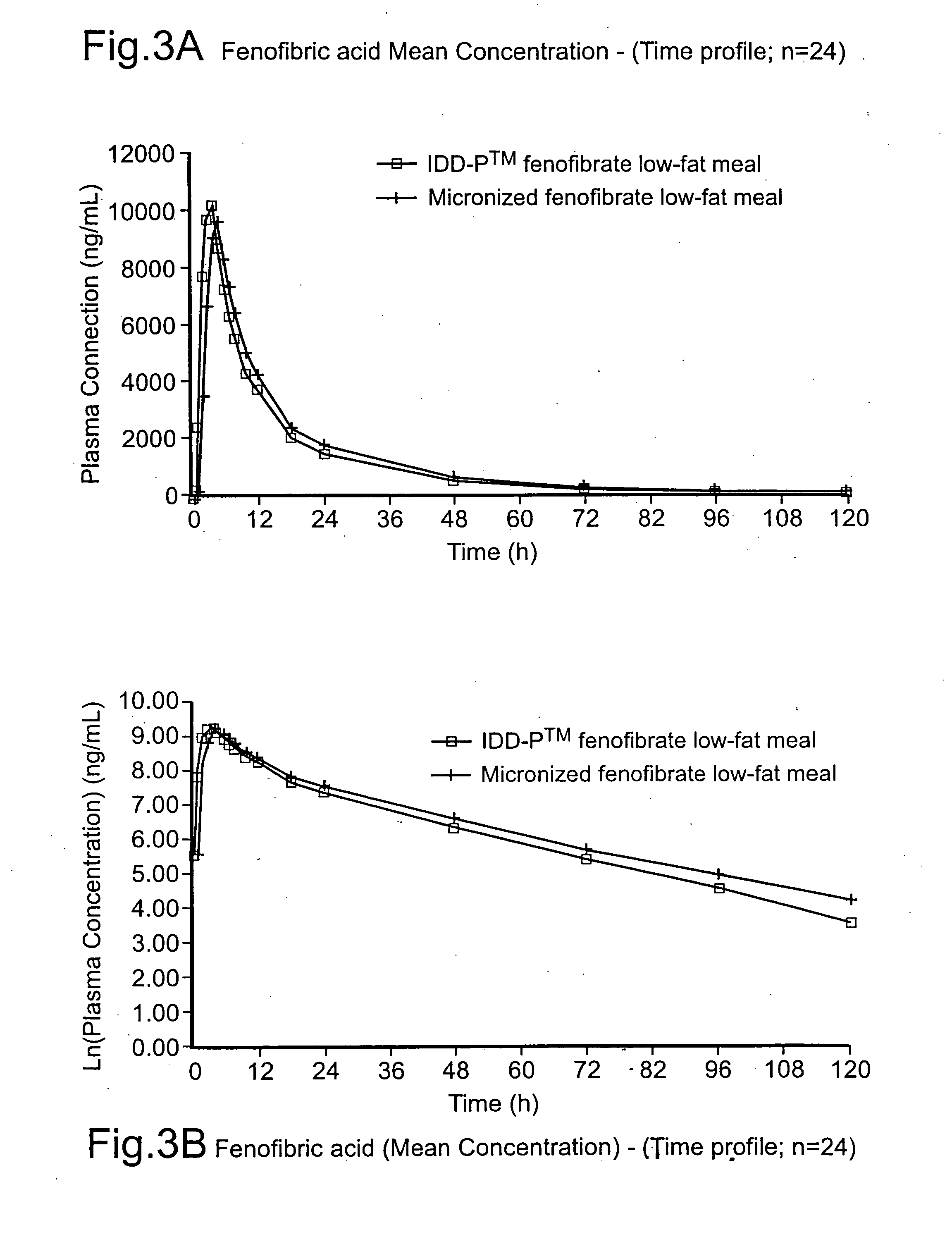
InactiveUS20060257494A1Improve bioavailabilityReduce the differencePowder deliveryBiocideCelluloseMicroparticle
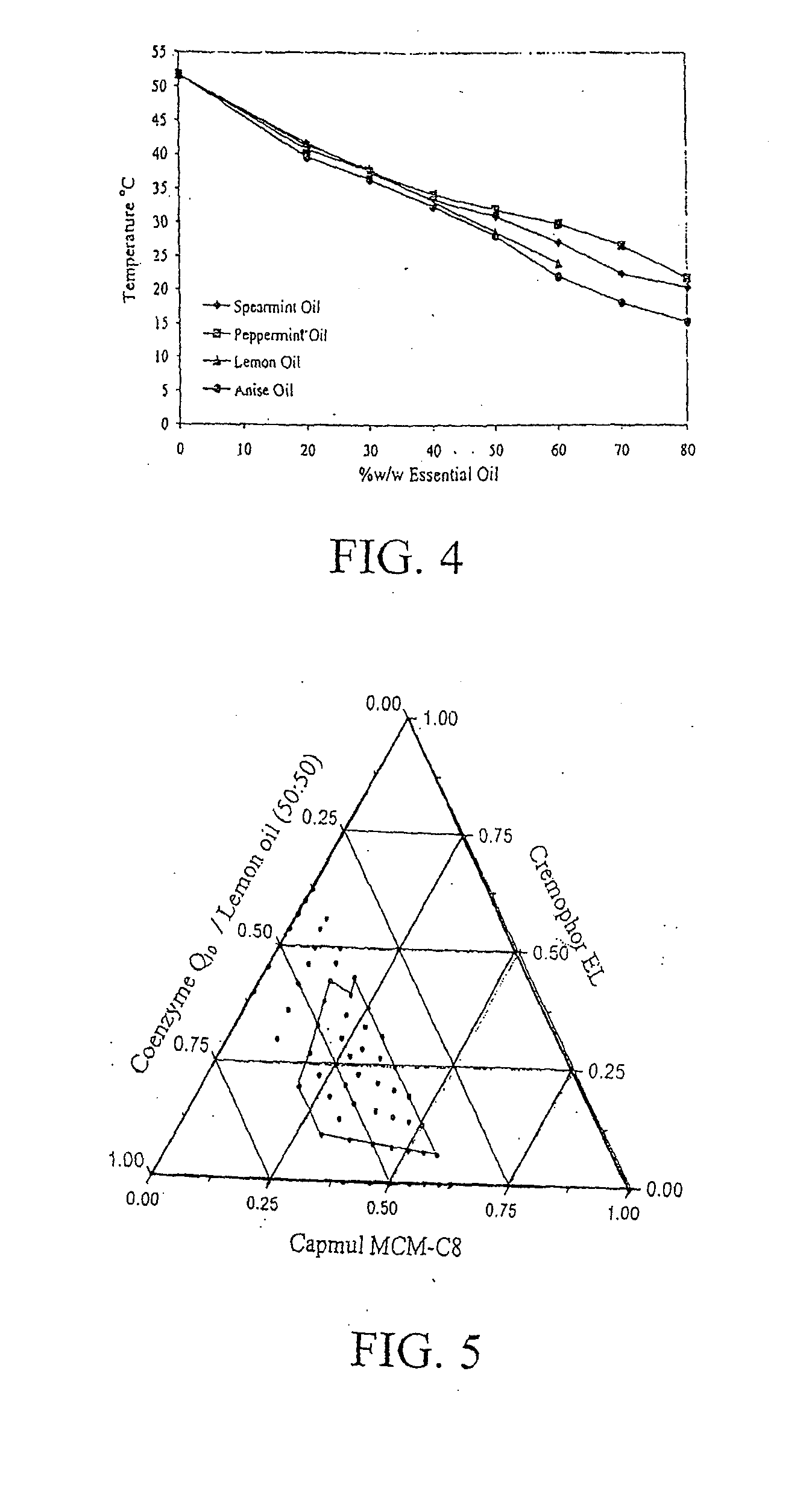
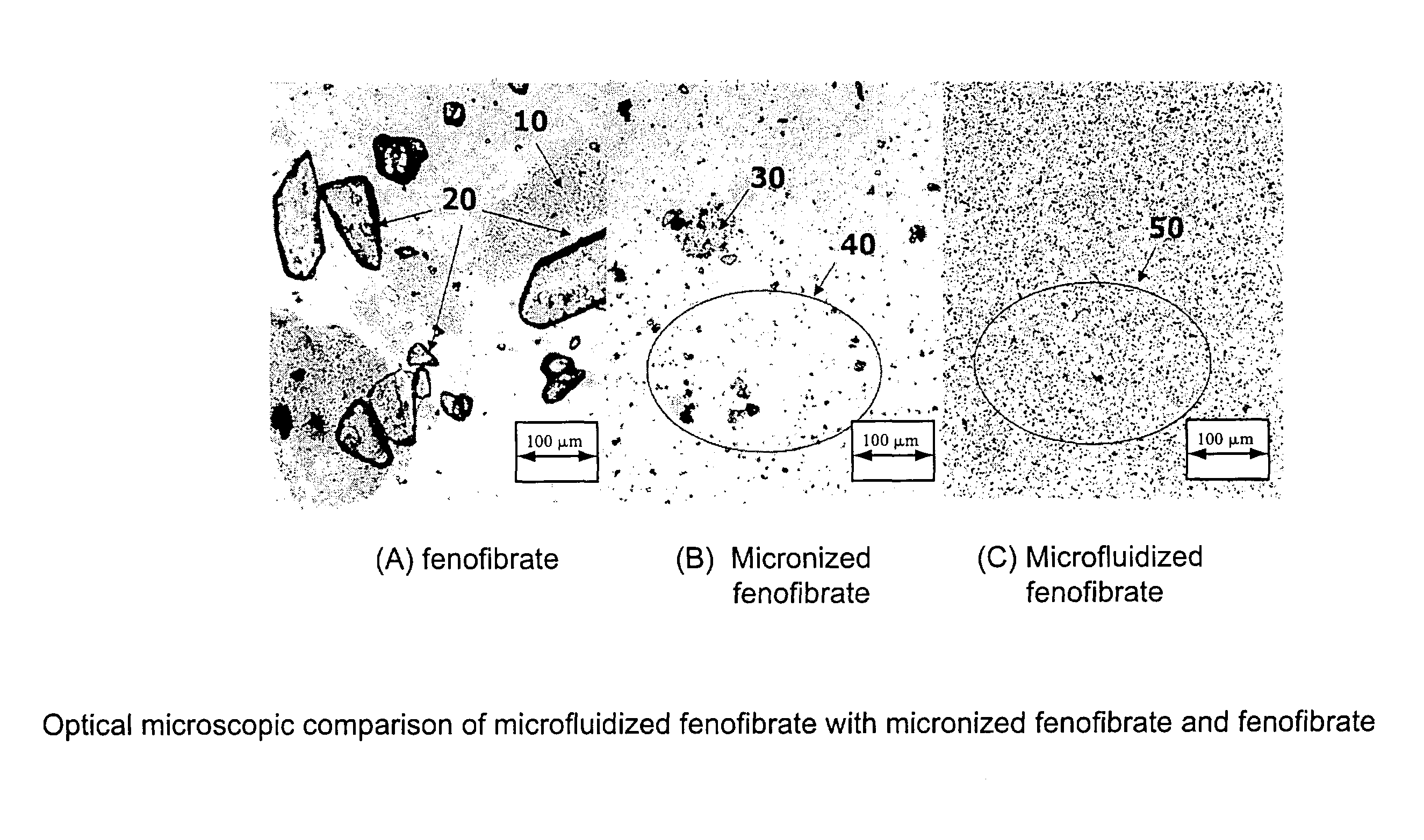
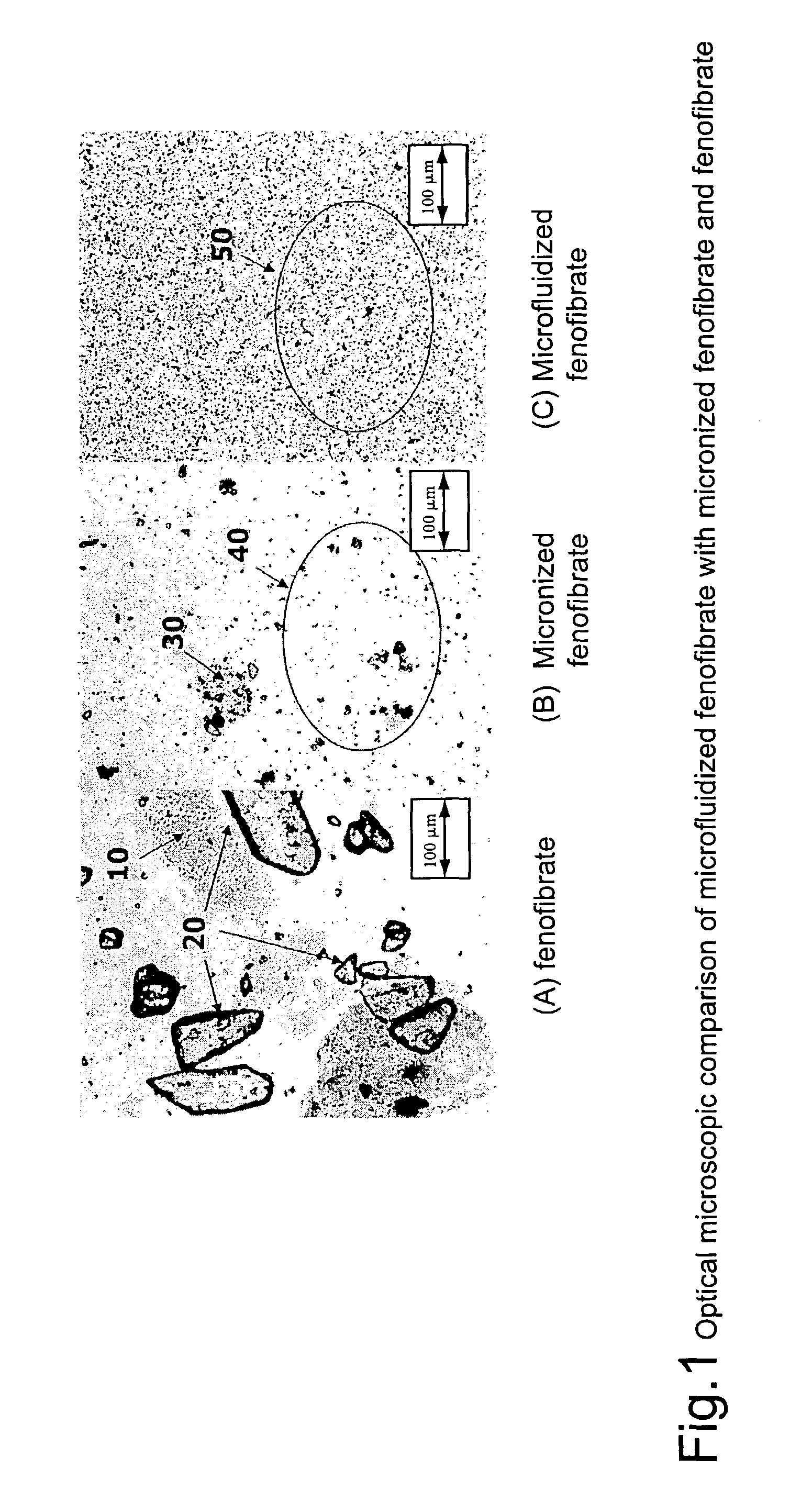
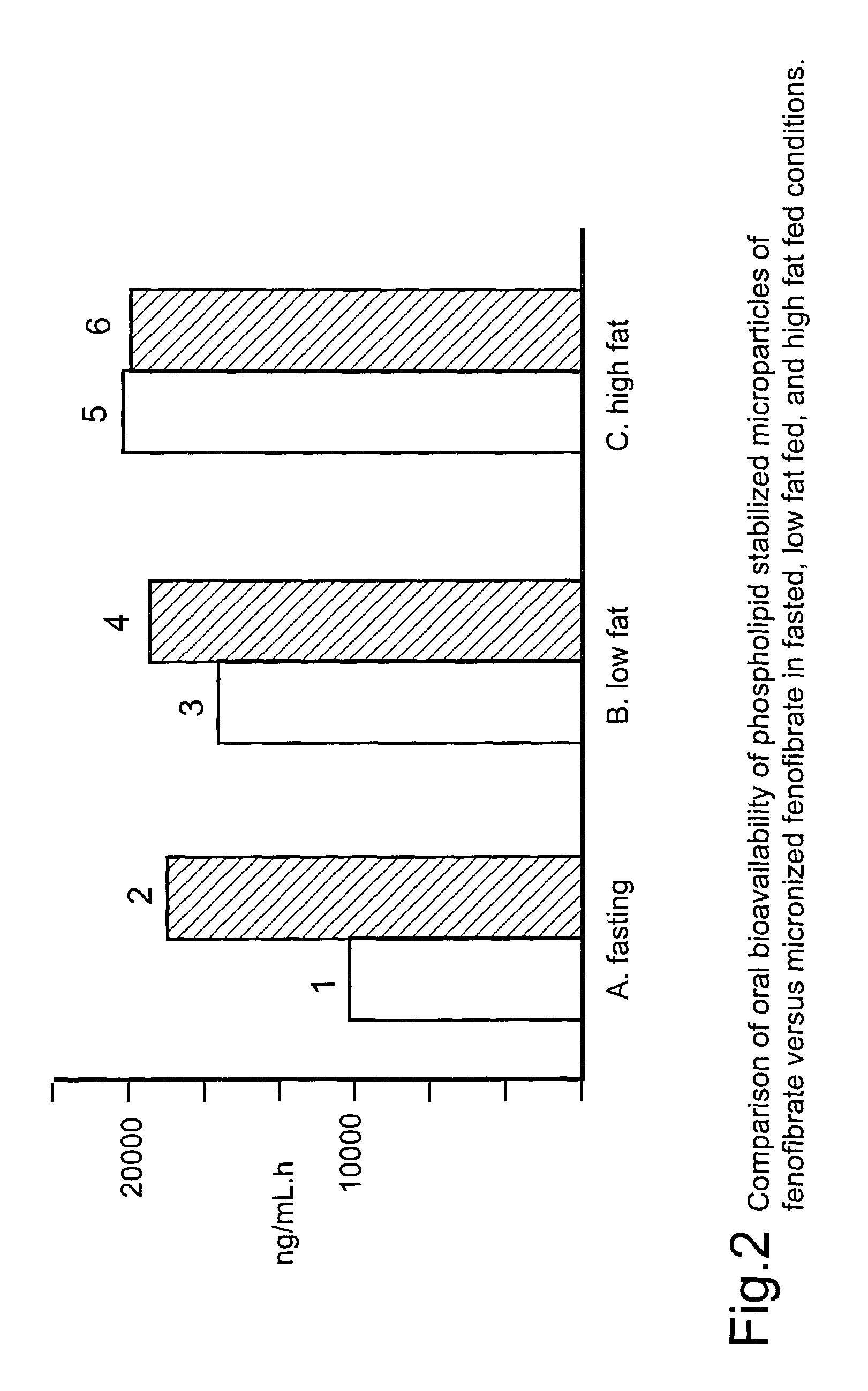
Disclosed is a pharmaceutically acceptable oral dosage form comprising fenofibrate, phospholipid, a buffer salt, a water-soluble bulking agent selected from maltodextrin, mannitol, and combinations thereof, a cellulosic additive, beads or crystals of a pharmaceutically acceptable water-soluble excipient support material, a polyvinylpyrrolidone or crospovidone, croscarmellose sodium, granular mannitol, sodium dodecyl sulfate, silicon dioxide, and a stearate, wherein the fenofibrate is in the form of microparticles, and wherein at least a portion of the phospholipid is coated on the surfaces of the fenofibrate microparticles, the phospholipid coated microparticles are embedded in a matrix comprising the water-soluble bulking agent, phospholipid that is not coated on the microparticles, the buffer salt and the cellulosic additive, and the matrix is coated on up to 100% of the surfaces of the beads or crystals of the excipient support material.
Owner:JAGOTEC AG
Cyclosporine emulsion and the preparing method
The present invention provides one kind of self-emulsified ciclosporin preparation and its preparation process. The self-emulsified ciclosporin preparation contains ciclosporin 5-25 wt%, vitamin E-TPGS 25-70 wt% and Pharmasolve 5-50 wt%. It has high stability, high medicine concentration, great capacity of self emulsifying, high bioactivity and easy taking, and may be used as orally taken liposoluble medicine.
Owner:SHANGHAI KAIZHAO PHARMA TECH
Cerebral apoplexy treating effervescence tablet and its preparation method
The invention relates to a cerebral apoplexy treating effervescence tablet and its preparation method, wherein the formula of the preparation comprises dried cerebral thrombus-treating concrete powder, cross bonding polyvinylpyrrolidone, mannitol, waterless lemon acid, sodium hydrogen carbonate, crystalline cellulose, miropowdered silica gel, magnesium stearate, talcum powder and Aspartame. The preparation employs a direct tabletting method, the bioavailability of the medicament can be improved, and the invention also has the advantages of convenient administration, reduced irritation to esophagus and gastrointestinal tract.
Owner:ZHEJIANG HISUN PHARMA CO LTD +1
Fullerene color-changing lipstick and preparation method thereof
InactiveCN111388357AEasy to spreadEasy to develop colorCosmetic preparationsMake-upCaprylyl GlycolPolymer science
The invention discloses a fullerene color-changing lipstick and a preparation method thereof, and relates to the technical field of skin care products. The fullerene color-changing lipstick includes the following components in parts by weight: 10-15 parts of water, 3-6 parts of white beeswax, 1-3 parts of candelilla wax, 4-6 parts of polyethylene, 2-4 parts of polyisobutylene, 1-2 parts of bis-diglyceryl polyacyladipate-2, 2-7 parts of phytosteryl oleate, 7-10 parts of ethylhexyl palmitate, 12-15 parts of cetyl ethyl hexanoate, 18-25 parts of polyglyceryl-2 diisostearate, 8-12 parts of ozokerite, 0.1-1 part of caprylyl glycol, 15-18 parts of polyglyceryl-2 triisostearate, 0.1-0.2 part of fullerene, 0.1-0.15 part of polyvinylpyrrolidone, 5-7 parts of butanediol, 2-3 parts of orange 4, 0.5-0.8 part of red 28, and 0.1-0.2 part of iron black. The fullerene color-changing lipstick provided by the invention is easy to spread, easily develops color, has a good makeup holding effect without lip balm primer, has anti-inflammatory effects, effectively alleviates cleft lip, has no obvious lip lines, modifies lip contour, makes a lip appear a full and soft state, and is very translucent.
Owner:广州市拉凯尔干细胞研究所
Mixture of new capable of degradation totally bone inside fixed polymer and cross-linking method
InactiveCN101199868AHigh mechanical strengthReduce manufacturing costSurgeryProsthesisInternal bone fixationPack material
Disclosed is a novel synthesis and cross-linking method which can completely degrading intraosseous fixed polymer. The invention adopts 1, 2-propylene glycol (or ethylene glycol ), dineopentyl alcohol neopentyl glycol, maleic anhydride, phthalic anhydride, glycin (or caprolactam) and cyclohexanol as raw material to synthesize an adjustable, innocuous, low-cost unsaturated polyesteramide, by a method of melt phase polycondensation and molecular chain end capping. By adding an amount of crosslinking agent (vinyl acetate or vinyl pyrrolidone) and initiating heat treatment after the accelerant crosslinking at room temperature, the completely degradable intraosseous fixed material with high strength can be produced. The unsaturated polyesteramide can also be developed into completely-degradable disposable tablewares, packing materials, flowerpots, medical supplies, drug-coating or basis materials such as delayed (controlled) release materials for capsules and drugs, glass fiber reinforced plastics etc.
Owner:HUNAN UNIV
Chiral monodentate phosphite ligands, its preparation method and uses
InactiveCN1951946AStable air and humidityEasy to store and operateOrganic reductionOrganic compound preparationDouble bondDichloromethane
The invention discloses a chiral monodentate phosphite ester ligand and preparing method, which comprises the following steps: a) placing chiral glycol and phsophorus trichloride in the reacting bottle; adding 2-methyl pyrrolidone; heating and refluxing until the chiral glycol is dissolved completely; decompressing to remove solvent; recrystallizing residual skellysolve B; obtaining the needed phosphorochloridous acid ester; b) dissolving phosphorochloridous acid ester in the dichloromethane; adding carbowax methyl ether or carbowax methyl ester and trimethylamine carrene solution at 0-5 deg.c; heating reacting liquid to 18-25 deg.c; stirring to react for 10-30h; filtering; removing solvent to obtain the product. The invention improves catalyzing activity, which makes TON at 10000.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Orally disintegrating tablet of gingko leaf and its preparation process
InactiveCN1593624AImprove stabilityEnhance pharmacological effectsUnknown materialsPill deliveryOrally disintegrating tabletGinkgo biloba
The invention discloses an orally disintegrating tablet of gingko leaf and its preparation process, characterized in that the tablet comprises the significant portions extracted from traditional Chinese medicinal ginkgo leaves and other medical use findings, the invention is also characterized that composite disintegrating tablet containing tetrahydroxy butane is employed, wherein the composite disintegrating tablet is obtained by combining tetrahydroxy butane with low substituted Hydroxypropylmethyl cellulose or sodium carboxymethylstarch or crossbond sodium carboxymethylstarch or insoluble cross bond polyvinylpyrrolidone by a finite proportion, the tetrahydroxy butane has the action of taste rectifying agent, thus reducing the consumption of medicinal findings in the preparation. Pharmacological experiment has shown that the disintegrating tablet has the advantages of quick disintegration, fast effect, and better pharmacological actions.
Owner:张晴龙
Preparation method for 4-azaspiro [2.4] heptane hydrochloride
The invention relates to a preparation method for 4-azaspiro [2.4] heptane hydrochloride and mainly aims to solve the technical problem that a suitable industrial synthesis method does not exist at present. The preparation method comprises the following steps of: firstly, preparing 4-benzyl-4-azaspiro [2.4] heptane by taking 1-benzylpyrrolidine-2-ketone and an ethylmagnesium bromide reagent as raw materials, and then, preparing the 4-azaspiro [2.4] heptane hydrochloride by using chloroethyl chloroformate. The reaction formula is shown as follows: the 4-azaspiro [2.4] heptane hydrochloride obtained by the preparation method is a useful midbody or product used for synthesizing plenty of drugs.
Owner:上海药明康德新药开发有限公司 +2
Unilateral mequindox injection and preparation method thereof
ActiveCN110893170AGuarantee product qualityAntibacterial agentsOrganic active ingredientsSodium salicylateVeterinary pharmaceuticals
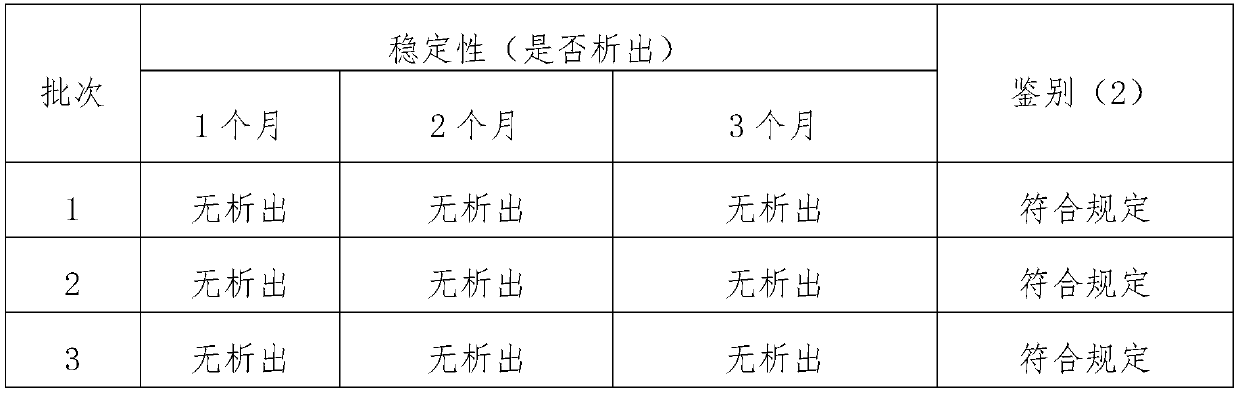
The present invention relates to the technical field of veterinary pharmaceuticals, and specifically relates to an unilateral mequindox injection and a preparation method thereof. The unilateral mequindox injection comprises the following feed compositions in mass concentrations: mequindox 18-22g / L; ethanol 180-220g / L; propylene glycol 90-110g / L; alpha-pyrrolidone 280-320g / L; and water for injection as a solvent. Compared to the prior art, sodium salicylate is removed from production process of the unilateral mequindox injection in the present invention, so as to obtain an injection product with stability comparable to an original injection, and maximum absorption can be detected at 242, 254 and 371 nm of wavelength, thereby ensuring quality of the product and meeting provisions in mequindox injection identification (2) section in Quality Standards for Veterinary Medicines, 2017 Edition, Chemicals Volume.
Owner:BEIJING LISHIDA PHARMA
Orally disintegrating tablet of 'Shengmai' and its preparation
The invention discloses an orally disintegrating tablet of 'Shengmai' and its preparation characterized in that the tablet comprises the significant portions extracted from traditional Chinese medicinal panax ginseng, ophiopogon root, schisandra fruit and other medical use findings, the invention is also characterized that composite disintegrating tablet containing tetrahydroxy butane is employed, wherein the composite disintegrating tablet is obtained by combining tetrahydroxy butane with low substituted Hydroxypropylmethyl cellulose or sodium carboxymethylstarch or crossbond sodium carboxymethylstarch or insoluble cross bond polyvinylpyrrolidone by a finite proportion, the tetrahydroxy butane has the action of taste rectifying agent, thus reducing the consumption of medicinal findings in the preparation. Pharmacological experiment has shown that the disintegrating tablet has the advantages of quick disintegration, fast effect, and better pharmacological actions.
Owner:张晴龙
Sildenafil citrate composition and preparation method thereof
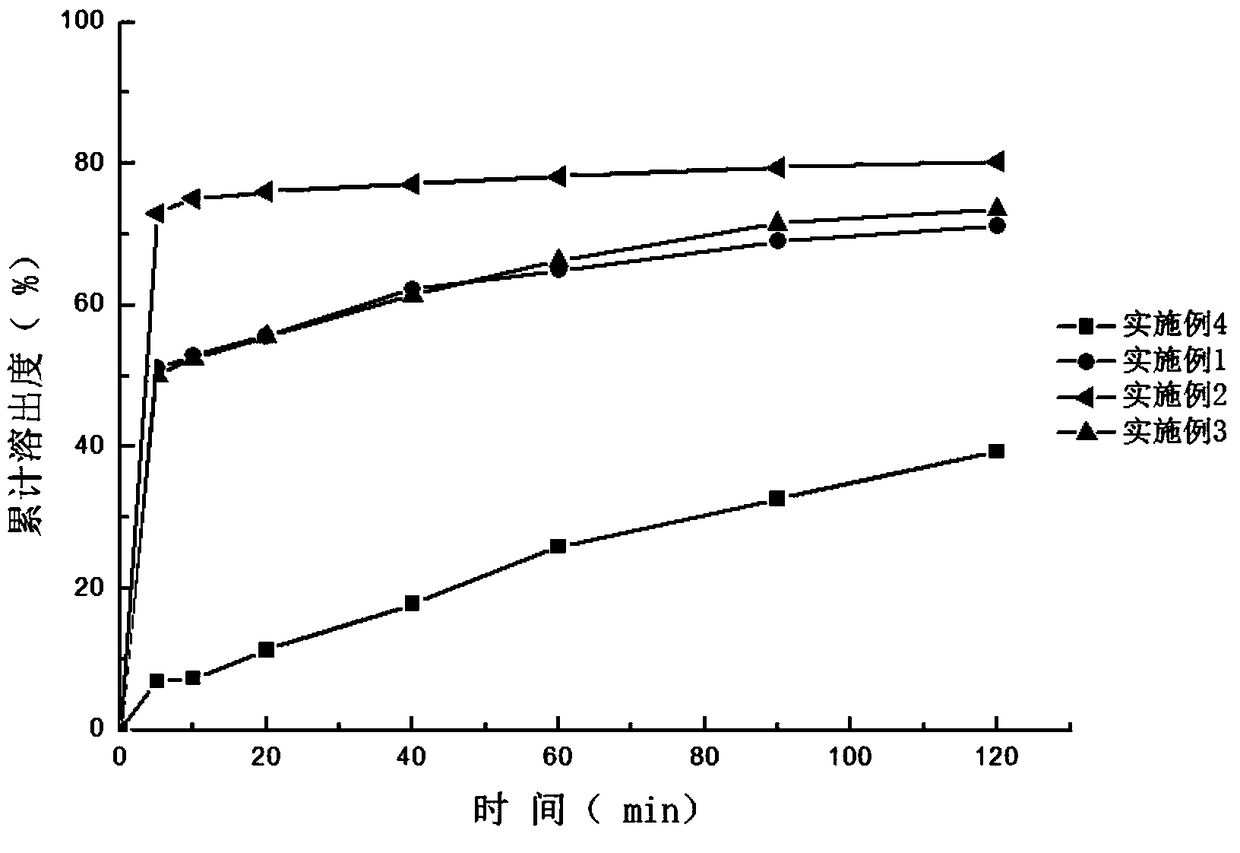
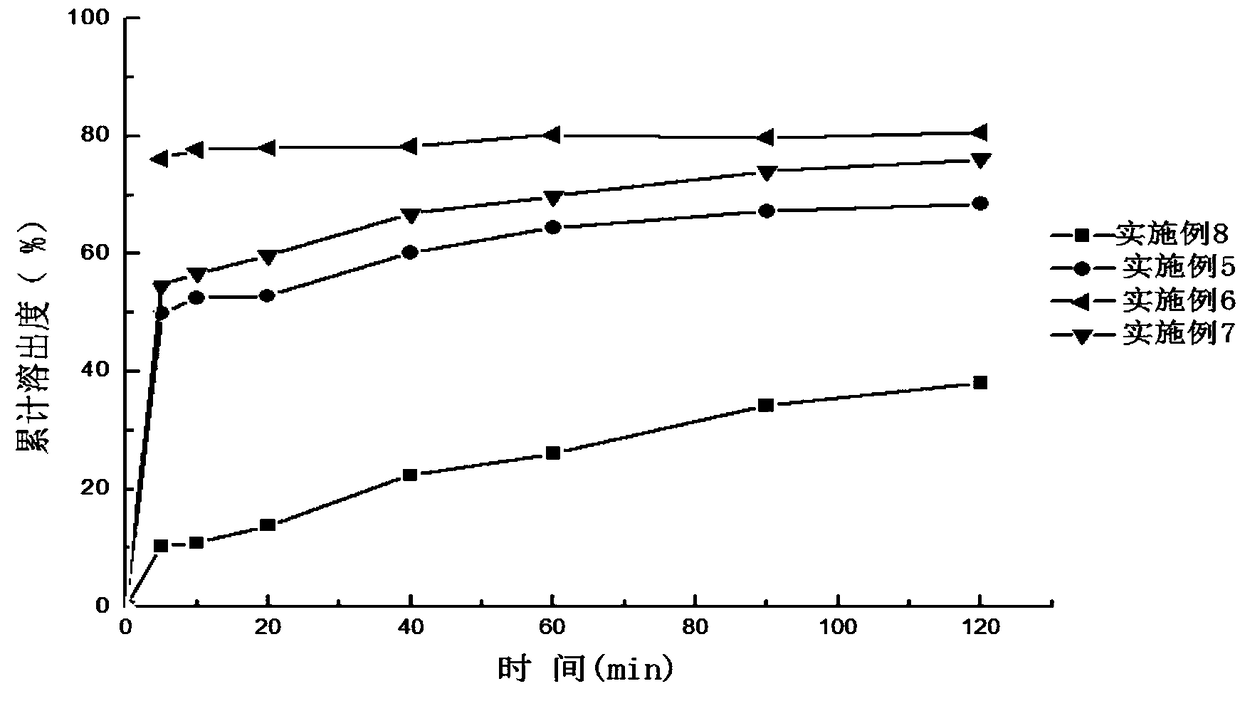
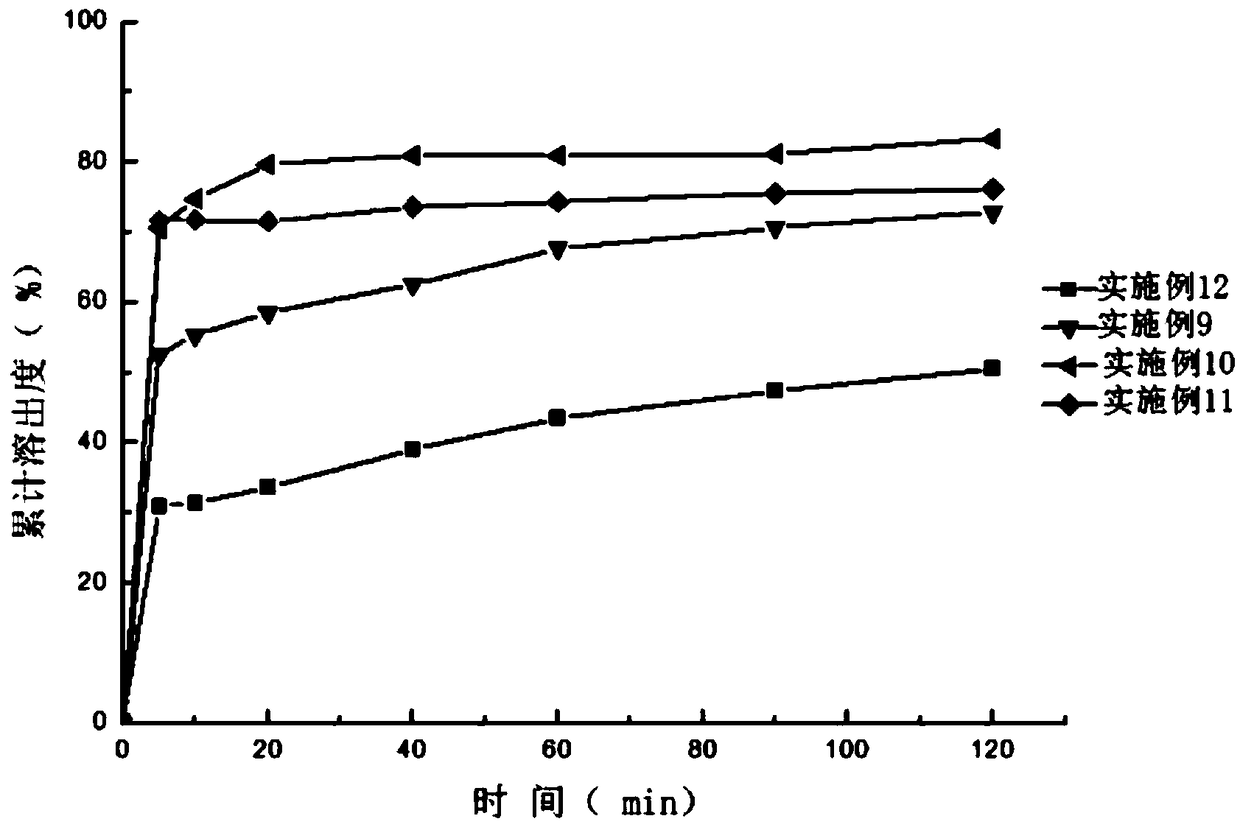
InactiveCN112206213ASimple prescriptionHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseDibutyl sebacate
The invention discloses a sildenafil citrate composition and a preparation method thereof, and belongs to the technical field of medicines. The sildenafil citrate composition takes sildenafil citrateas a main drug, and takes povidone K30, crospovidone, a vinyl pyrrolidone-vinyl acetate copolymer, xanthan gum, crosslinked carboxyl cellulose sodium, hydroxypropyl cellulose, triethyl citrate, tributyl citrate, dibutyl sebacate, diethyl phthalate, magnesium stearate and the like as auxiliary materials. Compared with those in the prior art, sildenafil citrate tablets or capsules obtained by adopting a hot-melt extrusion technology are simple and convenient to operate, environment-friendly, simple in prescription and suitable for large-scale production and application; and compared with preparations in the market, the obtained sildenafil citrate tablets or capsules are high in dissolution rate, good in stability, low in impurity content and high in clinical application safety.
Owner:广州汇元医药科技有限公司
Dimethylcurcumin solid dispersoid as well as preparation method and application thereof
InactiveCN108815121AEffective dissolutionPromote absorptionPowder deliveryMuscular disorderSolubilityDispersity
The invention discloses a dimethylcurcumin solid dispersoid as well as a preparation method and application thereof, and relates to the field of pharmaceutic preparations. The dimethylcurcumin solid dispersoid is formed by dimethylcurcumin and a water-soluble carrier material, wherein the water-soluble carrier material comprises PEG4000, PEG6000, polyvinylpyrrolidone (PVP K30), and poloxamer 188.The dimethylcurcumin solid dispersoid is prepared by using a melting method or a solvent method. An indissoluble drug of the dimethylcurcumin is prepared as the solid dispersoid. A dissolution rate, dispersity, solubility and bioavailability of the drug are improved. The solid dispersoid can be further prepared as solid preparations of a tablet, a capsule and the like. The dimethylcurcumin solid dispersoid is simple in preparation process, cheap in price, and easy to industrialization.
Owner:CHANGZHOU UNIV
Preparation method of compound anticoccidial medicine sulfachloropyrazine sodium solution
InactiveCN103070820ASimple production processImprove product qualityOrganic active ingredientsPharmaceutical delivery mechanismFormularyPyrazine
The invention relates to a preparation method of a compound high-efficiency anticoccidial medicine sulfachloropyrazine sodium and diaveridine solution. The solution is provided mainly for overcoming defects such as mixing difficulty, inconvenient dosing, inaccurate dose, and the like of existing powders. The solution comprises main components of: main medicines of sulfachloropyrazine sodium and diaveridine, and auxiliary materials of dimethyl sulfoxide, N-methylpyrrolidone, a cosolvent, and triethanolamine. A preparation process provided by the invention comprises the steps that: dimethyl sulfoxide, N-methylpyrrolidone, and sulfachloropyrazine sodium are dissolved in a liquid blending tank; diaveridine well dissolved by the cosolvent is added; and triethanolamine with a formulated dose is added. The method has the advantages of simple production process, stable product quality, simple feeding method, and accurate dose.
Owner:QINGDAO VLAND BIOTECH INC
Orally disintegrating tablet of 'Shuanghuanglian' and its preparation
InactiveCN1593622AEasy to storeImprove stabilityAntiviralsUnknown materialsOrally disintegrating tabletForsythia
The invention discloses an orally disintegrating tablet of 'Shuanghuanglian' and its preparation characterized in that the tablet comprises the significant portions extracted from traditional Chinese medicinal honeysuckle flower, baikal skullcap root, capsule of weeping forsythia and other medical use findings, the invention is also characterized that composite disintegrating tablet containing tetrahydroxy butane is employed, wherein the composite disintegrating tablet is obtained by combining tetrahydroxy butane with low substituted Hydroxypropylmethyl cellulose or sodium carboxymethylstarch or crossbond sodium carboxymethylstarch or insoluble cross bond polyvinylpyrrolidone by a finite proportion, the tetrahydroxy butane has the action of taste rectifying agent, thus reducing the consumption of medicinal findings in the preparation. Pharmacological experiment has shown that the disintegrating tablet has the advantages of quick disintegration, fast effect, and better pharmacological actions.
Owner:张晴龙
Dispersible tablet with gastrodia tuber for treating headache, and its testing method
InactiveCN100518798CPromote dissolutionFast absorptionNervous disorderPill deliveryGastrodiaClinical efficacy
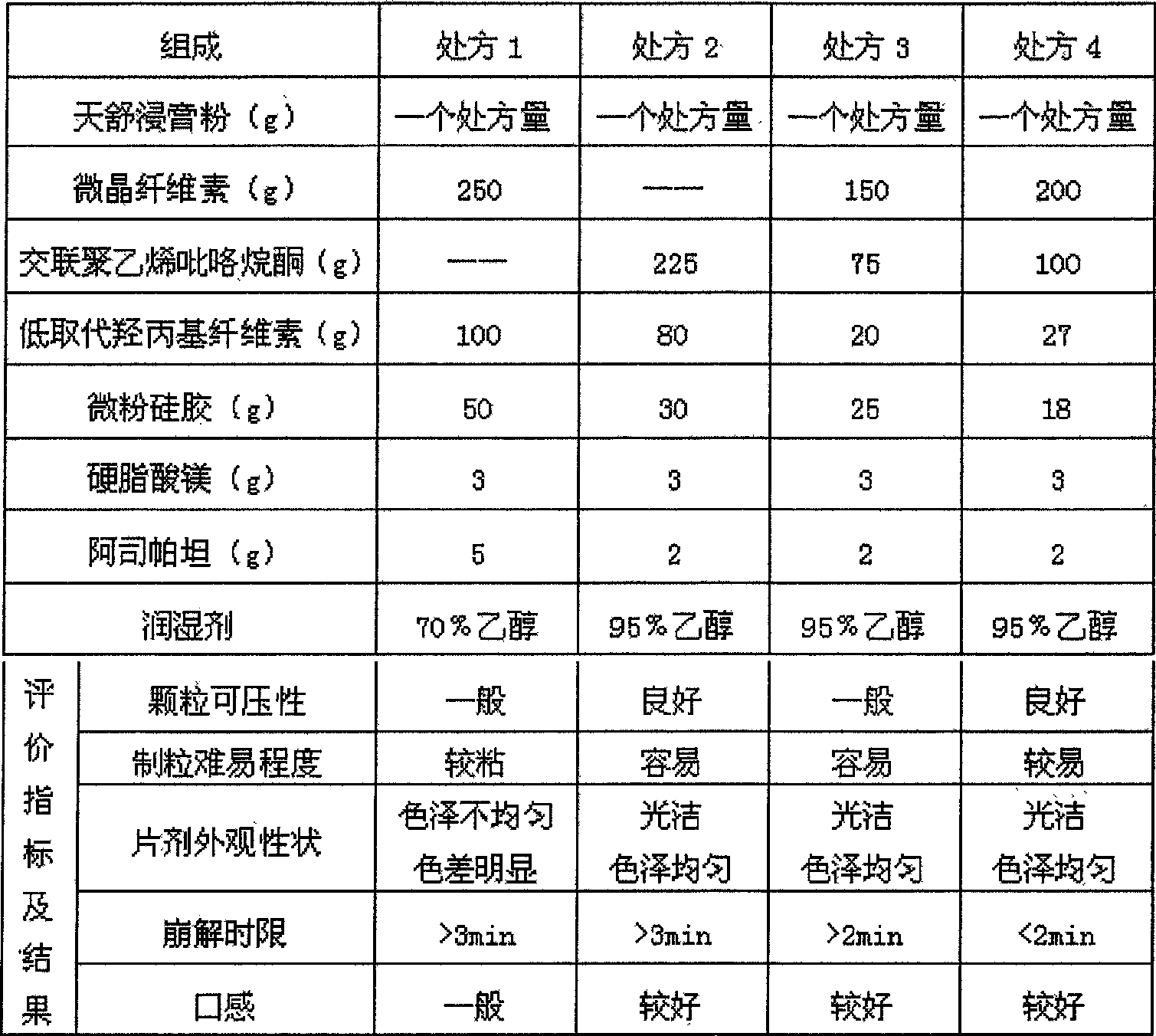
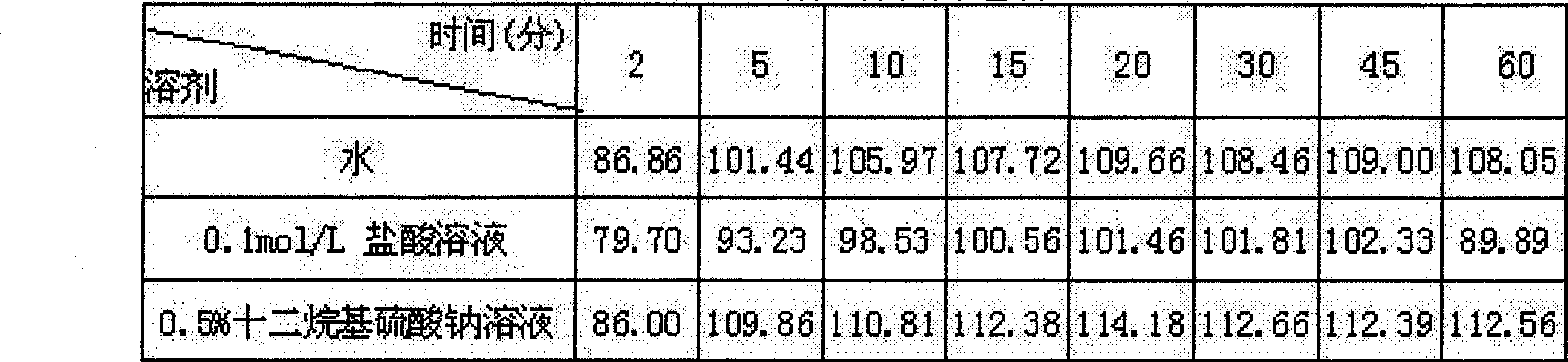
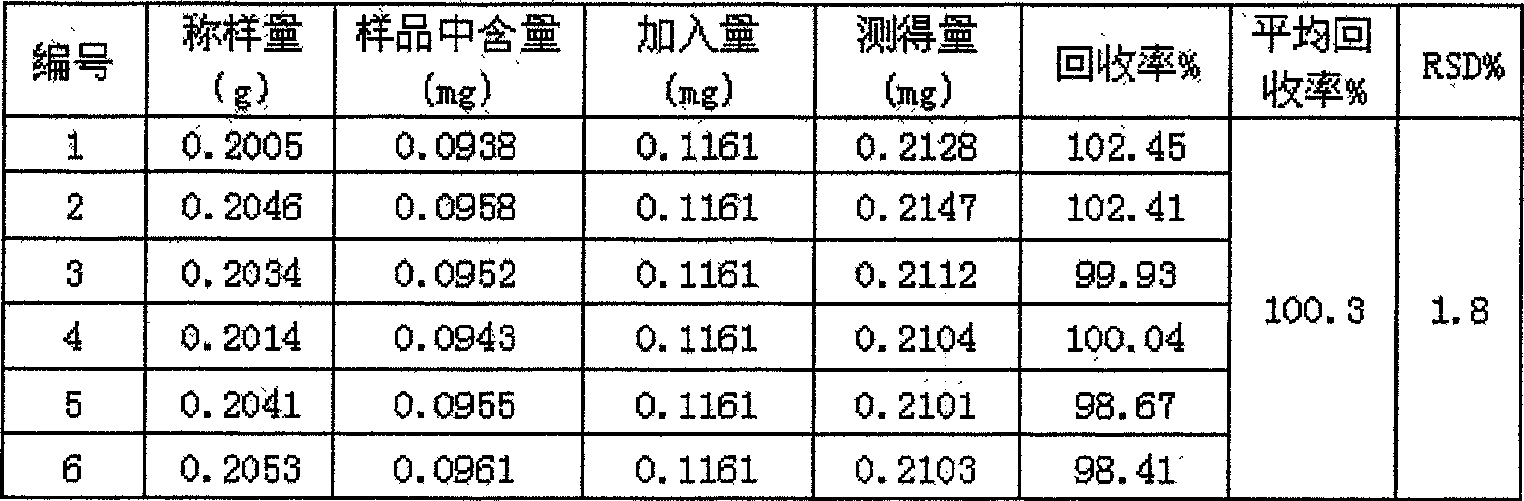
The invention provides a Tianshu dispersible tablet for treating headaches, a preparation method and a quality control method. Magnesium stearate and aspartame are prepared; compared with the prior art, the dispersible tablet of the present invention has the following advantages: the disintegration time is short, and the dispersion state is good; the medicine dissolves rapidly, absorbs quickly, and has high bioavailability; The production equipment is the same as that of ordinary tablets, which is suitable for large-scale industrial production; it is convenient to take and has various methods, especially suitable for the elderly, young and patients with difficulty swallowing; it is convenient to produce, carry, and transport, and the quality is stable. In addition, the quality control method of the present invention has high precision, good reproducibility and accurate measurement results, which can effectively guarantee the clinical curative effect of the preparation.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
Application of nano-drug targeting ferroptosis in treatment of acute kidney injury
PendingCN114699372AImprove stabilityGood biocompatibilityHeavy metal active ingredientsOrganic active ingredientsRenal Tubule EpitheliumPharmacology
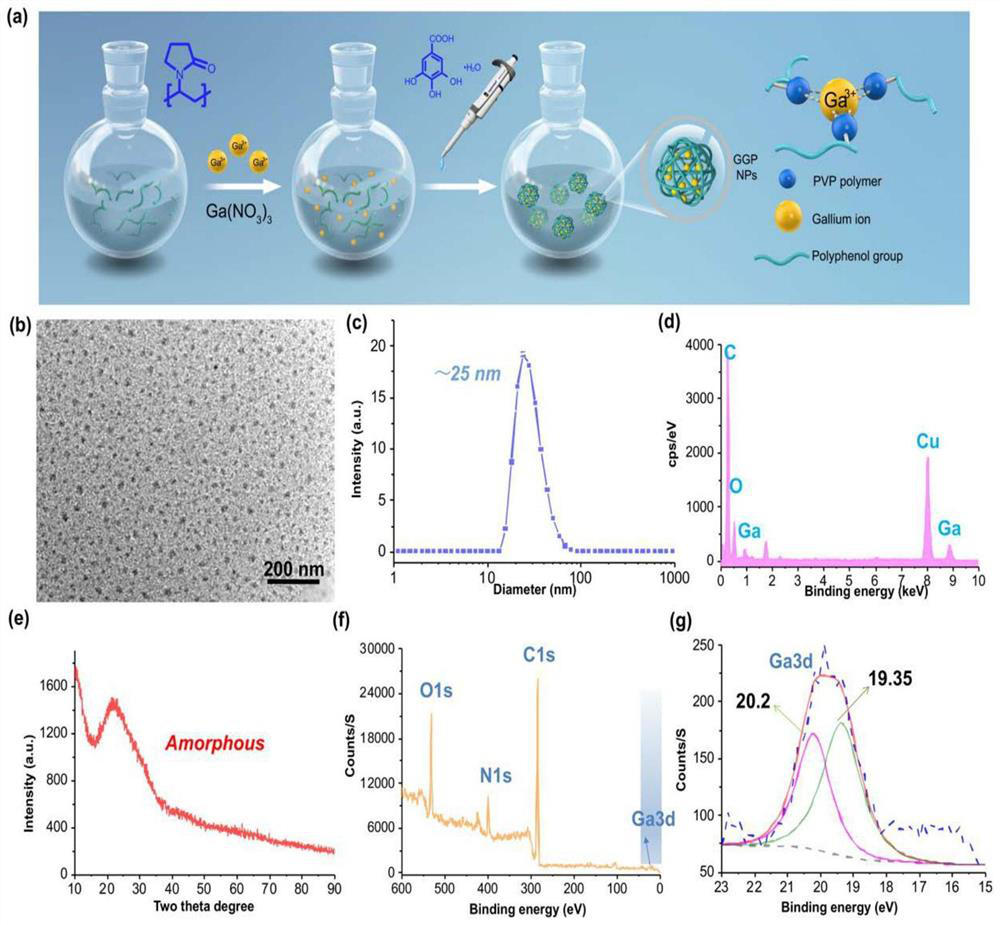
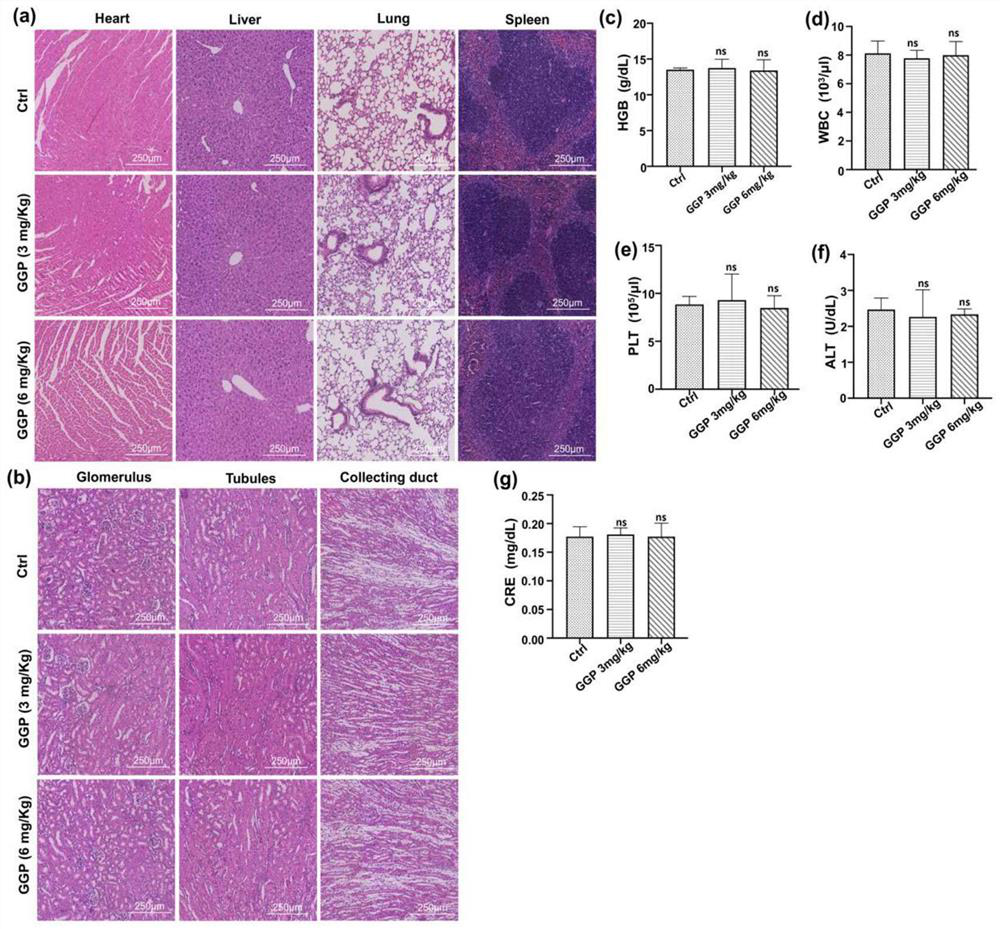
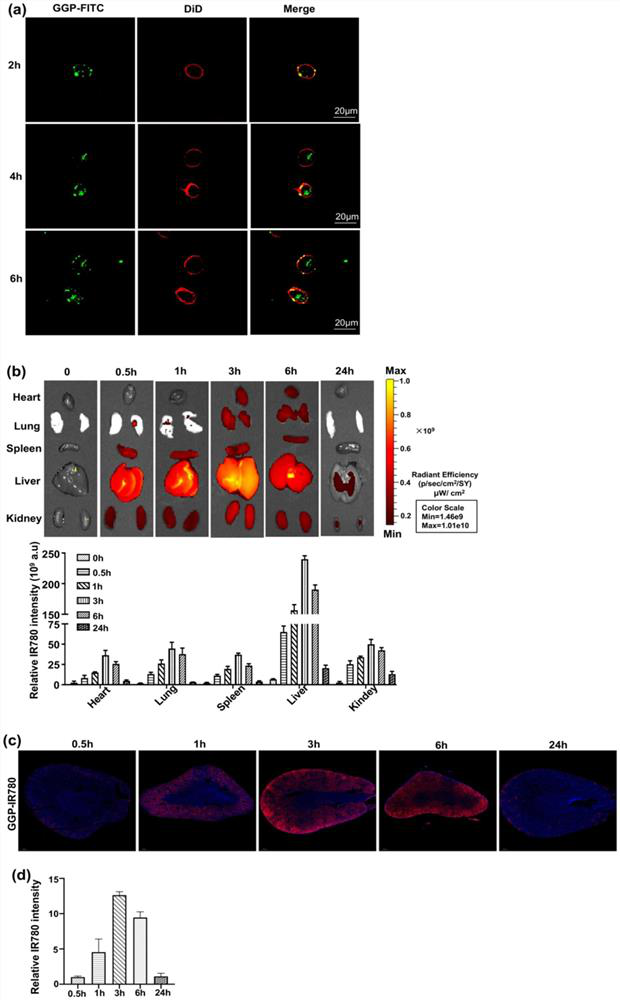
The invention discloses an application of a ferroptosis-targeting nano-drug in treatment of acute kidney injury. Gallium gallate polyvinylpyrrolidone nanoparticles (GGP NPs) are synthesized in the invention, and the nanoparticles have strong stability, excellent biocompatibility and effective iron substitution ability. The GGP NPs has the advantages that accumulation of free iron in cells and mitochondrial dysfunction are reduced, ferroptosis-mediated phenotypes including lipid peroxidation, NADPH (nicotinamide adenine dinucleotide phosphate) and glutathione levels and glutathione peroxidase 4 activity are inhibited, and accordingly the HK-2 cell ferroptosis induced by cis-platinum can be obviously inhibited. The GGP NPs treatment can significantly improve renal tubule injury and mitochondrial injury caused by cisplatin treatment or ischemia reperfusion injury. The GGP NPs synthesized by the invention may be an effective and promising candidate drug for AKI treatment.
Owner:THE FOURTH AFFILIATED HOSPITAL OF ZHEJIANG UNIV SCHOOL OF MEDICINE
Eutectic-Based Self-Nanoemulsified Drug Delivery System
InactiveUS20100166873A1High dissolution rateGood grinding agentBiocideMetabolism disorderMonoglycerideWater soluble drug
Owner:JARROW FORMULAS INC
Coated tablets
InactiveUS8586094B2Improve bioavailabilityReduce the differenceBiocidePowder deliveryCelluloseMicroparticle
Disclosed is a pharmaceutically acceptable oral dosage form comprising fenofibrate, phospholipid, a buffer salt, a water-soluble bulking agent selected from maltodextrin, mannitol, and combinations thereof, a cellulosic additive, beads or crystals of a pharmaceutically acceptable water-soluble excipient support material, a polyvinylpyrrolidone or crospovidone, croscarmellose sodium, granular mannitol, sodium dodecyl sulfate, silicon dioxide, and a stearate, wherein the fenofibrate is in the form of microparticles, and wherein at least a portion of the phospholipid is coated on the surfaces of the fenofibrate microparticles, the phospholipid coated microparticles are embedded in a matrix comprising the water-soluble bulking agent, phospholipid that is not coated on the microparticles, the buffer salt and the cellulosic additive, and the matrix is coated on up to 100% of the surfaces of the beads or crystals of the excipient support material.
Owner:PAUL ROYALTY FUND
Orally disintegrating tablets for treating functional dyspepsia
ActiveCN102302701BDigestive systemPharmaceutical non-active ingredientsOrally disintegrating tabletBaical Skullcap Root
The invention provides orally disintegrating tablets for treating functional dyspepsia, and the orally disintegrating tablets are prepared from gold thread extract, magnolia bark extract, acorus gramineus extract, processed pinellia tuber extract, baical skullcap root extract, dried tangerine peel extract, reed rhizome extract, oriental wormwood extract, raw coix extract, crosslinked polyvinylpyrrolidone used as a disintegrating agent, microcrystalline cellulose used as filler, magnesium stearate used as a lubricant and aspartame used as a flavoring agent. Patients suffering from spleen-stomach damp-heat syndrome functional dyspepsia are divided into a test group and a control group by adopting a multi-center random double-blind placebo control method, and 4 weeks are taken as a course of treatment. Results indicate that: the orally disintegrating tablets have excellent clinical curative effect to the functional dyspepsia spleen-stomach damp-heat syndrome, overcome the defects of the conventional Chinese medicinal preparation for treating the functional dyspepsia, and have safety and efficiency, low cost and convenient control.
Owner:BEIJING CHINESE MEDICINE HOSPITAL AFFILIATED CAPITAL MEDICAL UNIV
Cosmetic composition with gloss and long-lasting properties
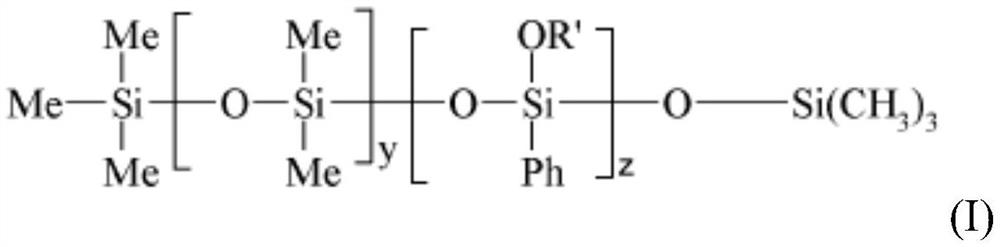
PendingCN114867459AHigh glossImprove featuresCosmetic preparationsHair cosmeticsPolymer scienceMeth-
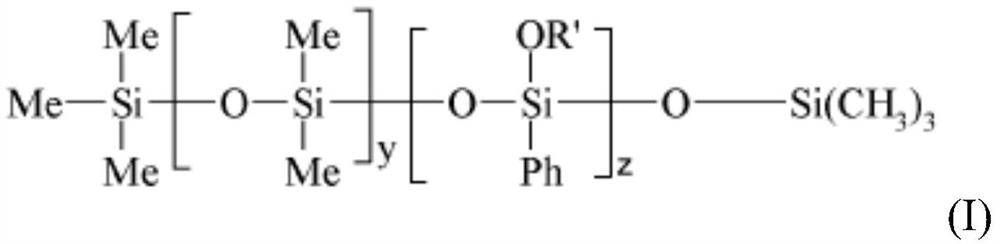
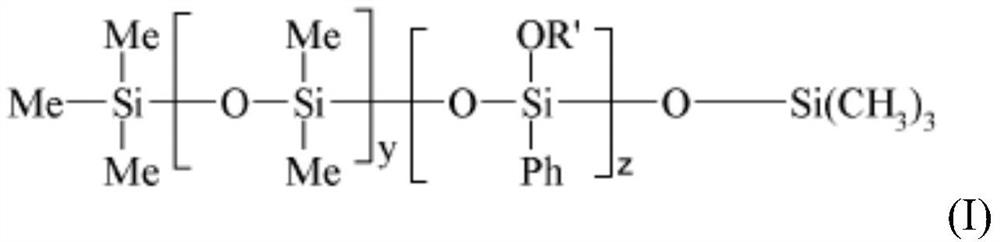
The present invention relates to a cosmetic composition comprising, in a physiologically acceptable medium, an oily phase and at least a) a phenyl silicone oil of formula (I) [Chemical Formula 1] (I) wherein-Me is methyl, Ph is phenyl, oR'represents a-OSiMe3 group,-y ranges from 1 to 1000, and-z ranges from 1 to 1000, b) a C8-C30 fatty alcohol, c) a branched dextrin ester, and d) advantageously a fat-soluble polymer selected from the group consisting of (i) acrylate polymers selected from the group consisting of silicon acrylate polymers, acrylate polymers comprising an alkyl chain of at least 10 carbon atoms, and copolymers of acrylates and acrylamides, the present invention relates to a cosmetic composition comprising (i) a copolymer of vinyl pyrrolidone (VP) and an olefin having at least 18 carbon atoms, (ii) a copolymer of vinyl pyrrolidone (VP) and an olefin having at least 18 carbon atoms, and (iii) mixtures thereof, and the use thereof for improving gloss and a more lasting cosmetic result of gloss and colour, in particular for lips. Preferably, the composition is in a semi-solid or solid form.
Owner:LVMH RECH
Orally disintegrating tablet of 'Baibaodan' and its preparation
The invention discloses an orally disintegrating tablet of 'Baibaodan'and its preparation, characterized in that the tablet comprises the significant portions extracted from traditional Chinese medicinal pseudo-ginseng, preserved Yunnan Kusnezoff monkshood root, Psammosilene tunicoides and other medical use findings, the invention is also characterized that composite disintegrating tablet containing tetrahydroxy butane is employed, wherein the composite disintegrating tablet is obtained by combining tetrahydroxy butane with low substituted Hydroxypropylmethyl cellulose or sodium carboxymethylstarch or crossbond sodium carboxymethylstarch or insoluble cross bond polyvinylpyrrolidone by a finite proportion, the tetrahydroxy butane has the action of taste rectifying agent, thus reducing the consumption of medicinal findings in the preparation. Pharmacological experiment has shown that the disintegrating tablet has the advantages of quick disintegration, fast effect, and better pharmacological actions.
Owner:张晴龙
A kind of preparation method of linezolid injection
ActiveCN110227063BFix non-compliant issuesReduce dosageAntibacterial agentsOrganic active ingredientsAluminium chlorideMethyl palmoxirate
The invention relates to a preparation method of linezolid injection, which belongs to the field of pharmaceutical preparations. The preparation method of the linezolid injection comprises: adding 400mL of water for injection, adding 2.3g of sodium acetate trihydrate and 1.2g of sodium chloride in sequence, placing it at a temperature of 0-5°C for 18-36 hours, and then Add 1.62g of sodium citrate and 0.85g-1.2g of citric acid in sequence, then add 50g of glucose, stir until completely dissolved to obtain a medicinal solution, add activated carbon, stir and absorb at 60-70°C for 15-25 minutes, then decarbonize to obtain The first filtrate; add 2g linezolid, 0.01g sodium bisulfite and 0.03g aluminum chloride to the first filtrate, stir and mix at 80-90℃, then add 50mL N-methylpyrrolidone, 10mL dimethyl sulfoxide and Add water for injection to 1000mL, stir to make the liquid medicine uniform, adjust the pH value of the liquid medicine to 4.4-5.0, filter to obtain the second filtrate; fill the second filtrate to obtain the linezolid injection. The quality of the linezolid injection prepared by the method is stable, and the pyrogen is qualified.
Owner:石药银湖制药有限公司
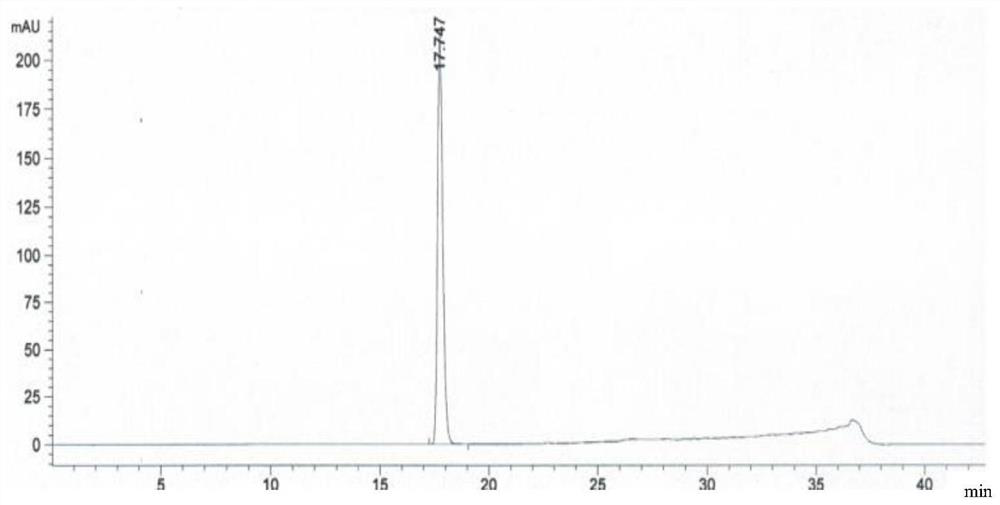
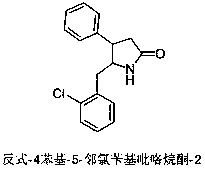
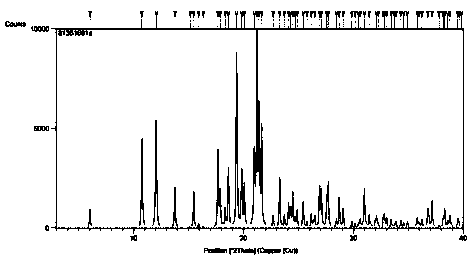
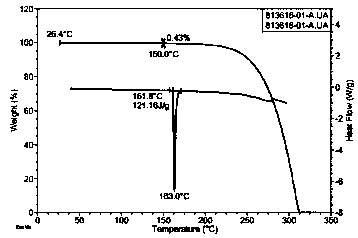
Crystal form of trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2
ActiveCN108997186AHigh crystallinityGood reproducibilityOrganic active ingredientsNervous disorderDiseaseBrain function
The invention relates to a new crystal form A of trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2, as well as a medicine composition comprising the crystal form and application thereof. The crystal form Acan be used for preparing medicines such as high-selective calcium antagonists and medicines for improving disordered brain function, resisting Alzheimer's disease and depression, and enhancing learning and memory capability. The preparation method of the trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 crystal form A is simple in operation and is controllable in crystallization process. The producthas high crystallization degree and good repeatability, and can be absorbed and utilized by body better.
Owner:北京优和康生物医药科技有限公司
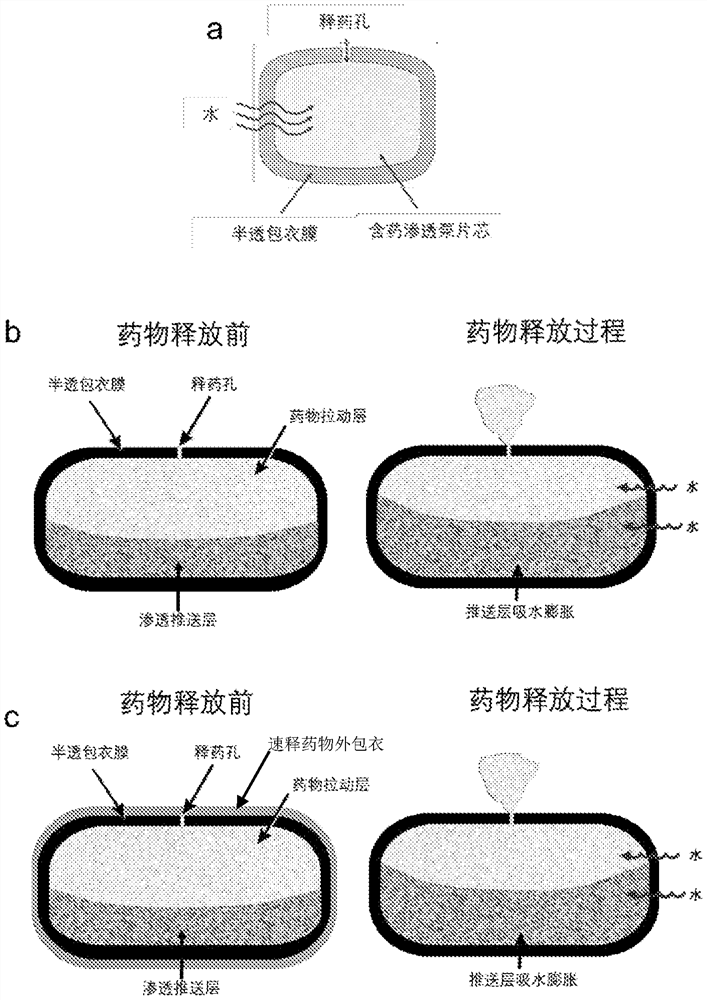
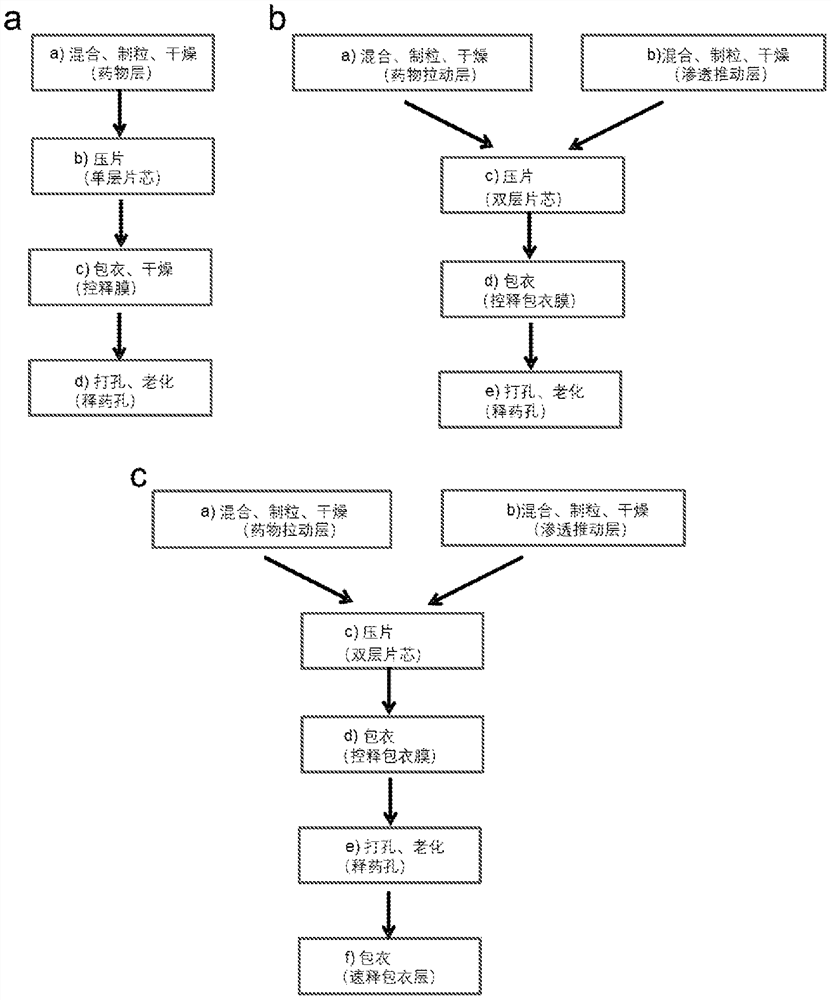
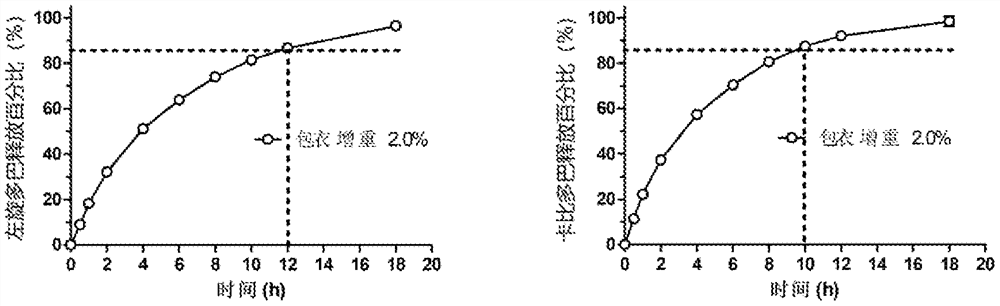
A kind of pharmaceutical composition and its preparation method and application
ActiveCN111526872BImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsCellulose acetateKetone
A pharmaceutical composition, an osmotic pump controlled-release drug delivery system containing the pharmaceutical composition and a preparation method thereof. The pharmaceutical composition comprises a tablet core and a coating film, the tablet core comprises a drug pulling layer, the coating film comprises 50-90% by weight of cellulose acetate and 10-50% by weight of copovidone, and the copolyvidone Ketones are prepared by polymerizing vinylpyrrolidone and vinyl acetate in a molar ratio of 40:60‑80:20.
Owner:SHANGHAI WD PHARM CO LTD
A kind of moisturizing facial mask and preparation method thereof
ActiveCN112294705BGive full play to the moisturizing effectGood moisturizing effectCosmetic preparationsToilet preparationsCarrageenanBetaine
The present invention relates to a moisturizing facial mask. The composite moisturizing agent is added to the facial mask of the present invention, and its composition and dosage are (wt%): sodium hyaluronate 0.01-5.0, wrinkled carrageenan 0.01-5.0, sodium polyglutamate 0.01-5.0, glycerin 0.001-40.0 , L-serine 0.01-2.0, L-proline 0.00-2.0, L-glutamic acid 0.0-0.3, betaine 0.001-15.0, urea 0.0-20.0, sodium pyrrolidone carboxylate 0.001-15.0, trehalose 0.0-10.0 , preservative 0.0‑1.0, allantoin 0.001‑0.5, pH regulator 0.001‑5.0. The moisturizing facial mask of the invention has film-forming properties, can exert a lasting and efficient moisturizing effect, and has wide application prospects.
Owner:BEIJING ACAD OF TCM BEAUTY SUPPLEMENTS
Pramipexole tablet preparation method, tablet prepared therethrough, and application of tablet
ActiveCN103961325ALow content of related substancesSimple manufacturing methodOrganic active ingredientsNervous disorderPramipexoleMagnesium stearate
The invention provides a pramipexole tablet preparation method. The method comprises the following steps: suspending starch in a solvent, fully swelling, dispersing and stirring, filtering, and drying; sieving pramipexole, starch, mannitol, silica and magnesium stearate, fully mixing pramipexole with starch according to an equivalent progressive maximum process; and sequentially adding mannitol and an ethanol solution of polyvinylpyrrolidone, granulating, drying, and adding magnesium stearate and silica. The invention also provides a pramipexole tablet prepared through the preparation method, and an application of the pramipexole tablet in the preparation of medicines for treating the Parkinson's disease and restless legs syndrome. The content of related substances in the pramipexole tablet is less than 0.5%. The pramipexole tablet prepared through the method contains few types of the related substances, and has an obviously reduced content of total impurities.
Owner:NANJING SANHOME PHARM RES & DEV CO LTD
Polygonum multiflorum nutrient tablet and making method thereof
InactiveCN103099210BImprove immunityAvoid wastingMetabolism disorderPharmaceutical delivery mechanismBiotechnologyCellulose
The invention relates to a Polygonum multiflorum nutritional tablet and a production method thereof, which are made of the following raw materials in parts by weight: 200-600 parts of Polygonum multiflorum, 70-100 parts of vitamin C, 0.2-1 parts of vitamin B, 1-2 parts of vitamin E, hydroxypropyl 1-5 parts of methyl cellulose, 10-20 parts of polyvinylpyrrolidone, 8-16 parts of light aluminum hydroxide, 1-8 parts of magnesium stearate. The preparation method includes the following steps: (1) sifting the micronized powder of Polygonum multiflorum to 180-500 mesh for standby; (2) mixing vitamin E and light aluminum hydroxide through a 100-mesh sieve for standby; Grind the powder in a ball mill; (4) make slurry with hydroxypropyl methylcellulose; make slurry with polyvinylpyrrole; mix the two evenly and make slurry for later use; (5) make tablet. The combination of Chinese and Western medicines in the invention can greatly enhance the pharmacological activity; the ultrafine pulverized or finer medicine has stronger pharmacokinetic activity and higher bioavailability.
Owner:江门酵敬堂生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

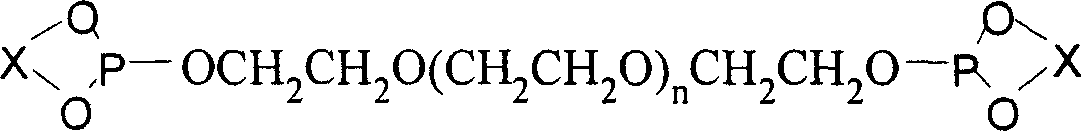
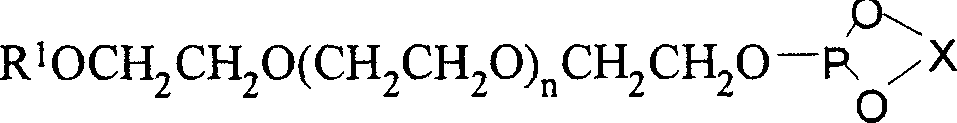
![Preparation method for 4-azaspiro [2.4] heptane hydrochloride Preparation method for 4-azaspiro [2.4] heptane hydrochloride](https://images-eureka.patsnap.com/patent_img/68be0e59-0e80-4297-8275-b6dbbf9ea365/142608DEST_PATH_IMAGE001.PNG)
![Preparation method for 4-azaspiro [2.4] heptane hydrochloride Preparation method for 4-azaspiro [2.4] heptane hydrochloride](https://images-eureka.patsnap.com/patent_img/68be0e59-0e80-4297-8275-b6dbbf9ea365/318374DEST_PATH_IMAGE002.PNG)
![Preparation method for 4-azaspiro [2.4] heptane hydrochloride Preparation method for 4-azaspiro [2.4] heptane hydrochloride](https://images-eureka.patsnap.com/patent_img/68be0e59-0e80-4297-8275-b6dbbf9ea365/56654DEST_PATH_IMAGE003.PNG)