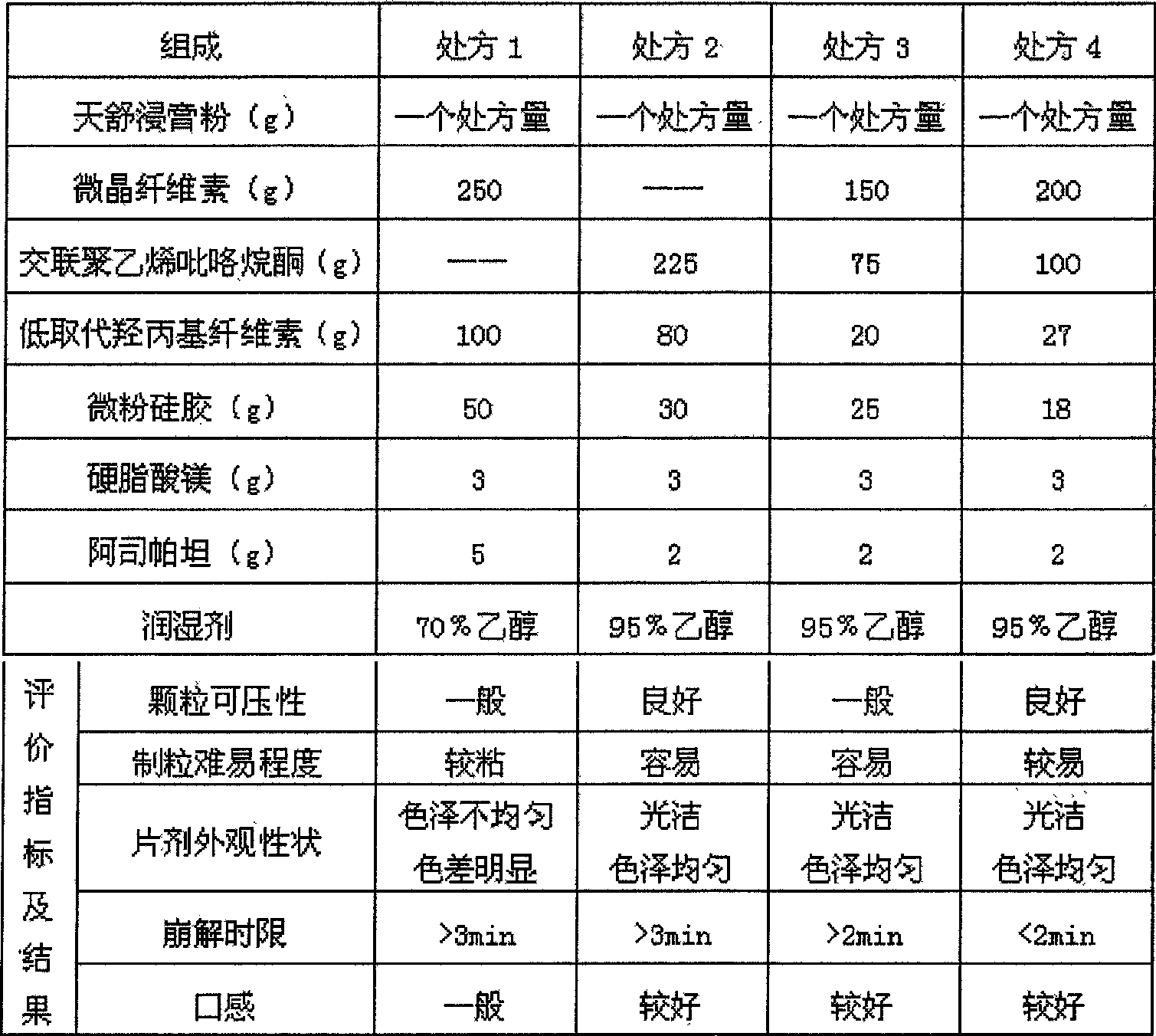
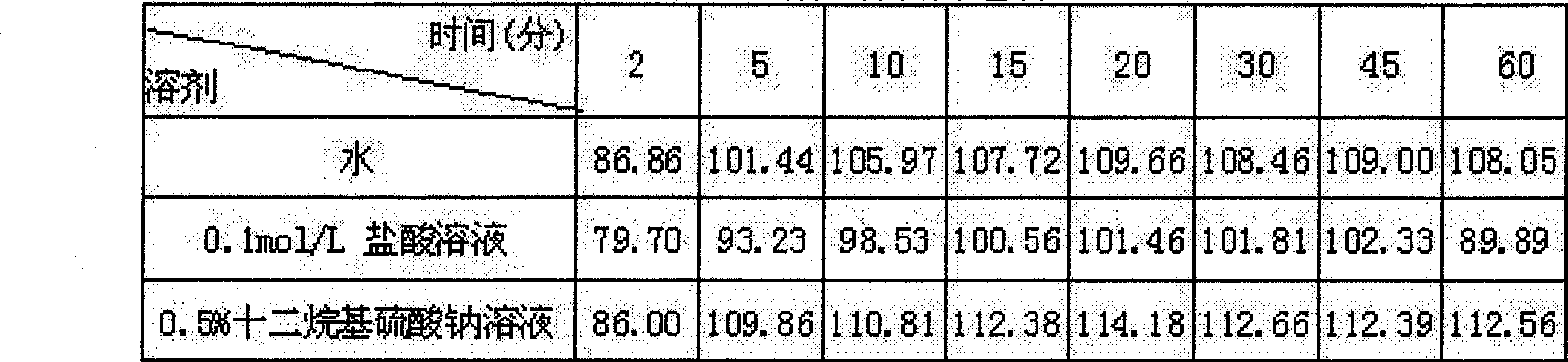
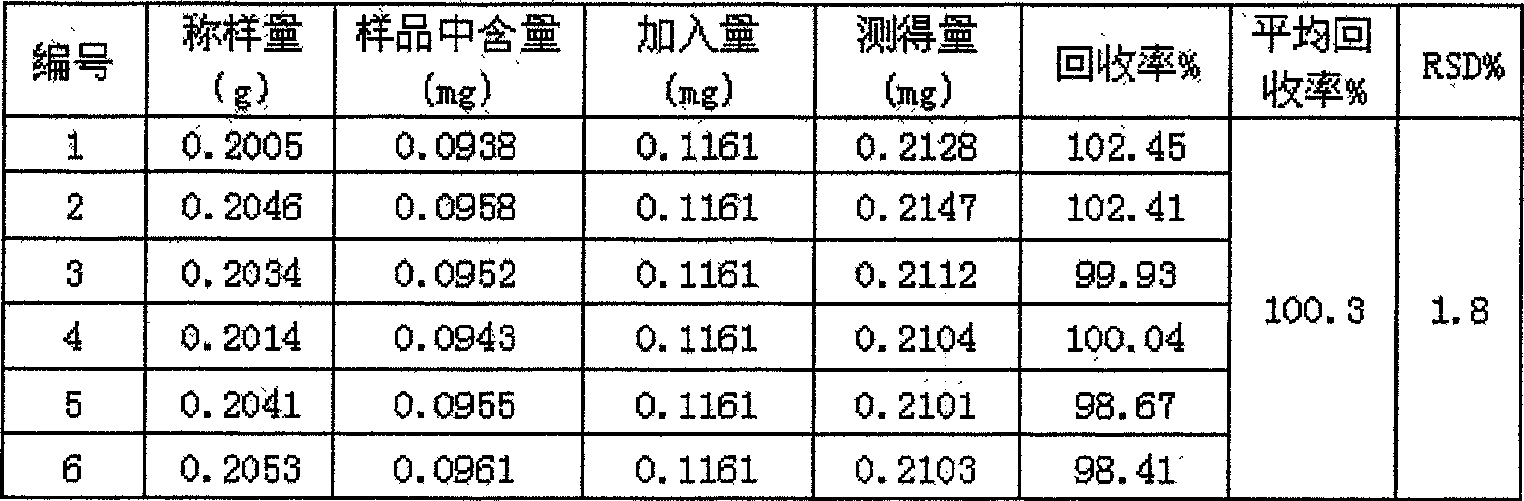
Dispersible tablet with gastrodia tuber for treating headache, and its testing method
A detection method and technology for dispersible tablets, which are applied in pharmaceutical formulations, medical preparations containing active ingredients, plant/algae/fungus/moss components, etc., can solve the problem of high local drug concentration, achieve rapid drug dissolution, convenient transportation, High bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0196] Embodiment 2 of the present invention: described quality control method comprises the following contents:
[0197] Properties: The drug is brown to brown tablets; it has a special aroma and a slightly bitter taste;
[0198] Identification: (1) Take 3 tablets of this preparation, grind them finely, add 15ml of water to dissolve, filter, shake the filtrate with 25ml of diethyl ether, discard the diethyl ether solution, shake the water layer with 20ml of n-butanol, separate and take n-butanol Alcoholic liquid, evaporated to dryness, add methanol 1ml to the residue to dissolve, as the test solution; take another gastrodin reference substance, add methanol to make a solution containing 0.5mg per 1ml, as the reference substance solution; according to the Chinese Pharmacopoeia 2005 edition one Appendix VIB Thin-layer chromatography test, draw 5 μl of each of the above two solutions, and place them on the same silica gel G thin-layer plate with sodium carboxymethylcellulose as ...
Embodiment 3
[0209] Embodiment 3 of the present invention: quality control method may comprise the following content:
[0210] Properties: The drug is brown to brown tablets; it has a special aroma and a slightly bitter taste;
[0211]Identification: Take 3 tablets of this preparation, grind them finely, add 15ml of water to dissolve, filter, shake the filtrate with 25ml of ether, discard the ether solution, shake the water layer with 20ml of n-butanol, separate the n-butanol solution, Evaporate to dryness, add 1ml of methanol to the residue to dissolve, and use it as the test solution; take another gastrodin reference substance, add methanol to make a solution containing 0.5mg per 1ml, and use it as the reference substance solution; according to the Chinese Pharmacopoeia 2005 edition one appendix VIB thin For layer chromatography test, draw 5 μl of each of the above two solutions, and spot them on the same silica gel G thin-layer plate with sodium carboxymethylcellulose as the binder, and...
Embodiment 4
[0218] Embodiment 4 of the present invention: quality control method may comprise the following content:
[0219] Properties: The drug is brown to brown tablets; it has a special aroma and a slightly bitter taste;
[0220] Identification: Take 2 tablets of this preparation, grind finely, add 20ml of ether, heat and reflux for 1 hour, filter, evaporate the filtrate to dryness, add 2ml of ethyl acetate to the residue to dissolve, and use it as the test solution; Prepare the reference medicinal material solution according to the method; according to the Chinese Pharmacopoeia 2005 edition one appendix VIB thin-layer chromatography test, draw each 2 μ l of the above two solutions, respectively spot on the same silica gel G thin-layer plate, with n-hexane:ethyl acetate=9 : 1 is a developing agent, unfolded, taken out, dried, and inspected under an ultraviolet lamp, in the chromatogram of the test product, on the position corresponding to the chromatogram of the contrast medicinal ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com