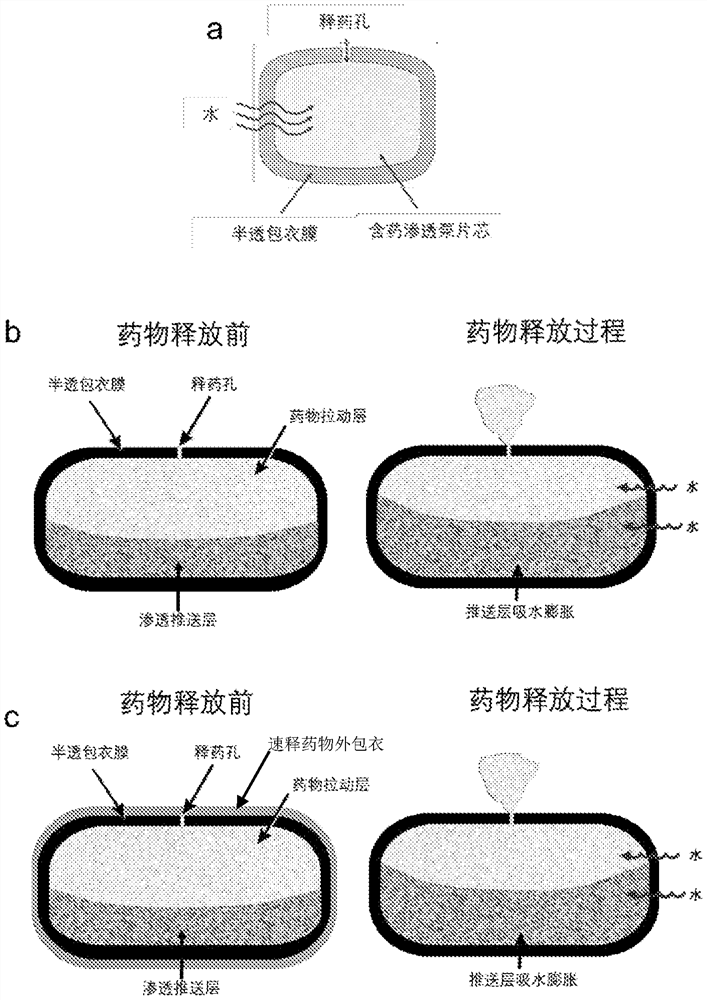
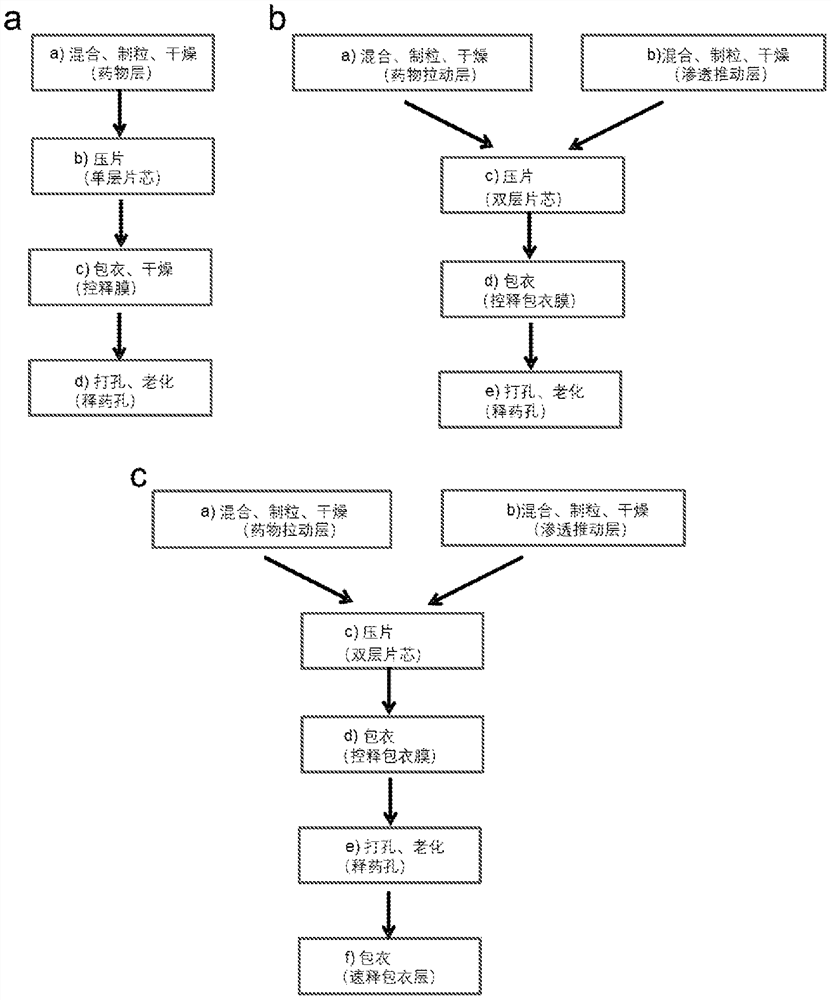
A kind of pharmaceutical composition and its preparation method and application
A composition and drug technology, applied in the field of biomedicine, can solve the problems of inability to provide, instability, etc., and achieve the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] A dosage form for delivering the beneficial agents levodopa and carbidopa to the oral cavity is manufactured as follows: First, a tablet core is prepared comprising 40.0 wt% levodopa (LD), 10.8 wt% carbidopa monohydrate (CD), 20.0Wt% microcrystalline cellulose, 18.7Wt% mannitol, 5.0Wt% hydroxypropyl methylcellulose (HPMC E5) and 5.0Wt% citric acid, by weight percentage, respectively Pass through a 40-mesh stainless steel sieve, then mix with pure water and granulate until a uniform wet material is formed; the wet material passes through a 20-mesh stainless steel sieve and dry at 80°C for 2 hours; pass the dried granules through a 18-mesh stainless steel sieve, Then mixed with 0.5wt% magnesium stearate.
[0136] Next, using a tablet press, 500 mg of the drug core granules were compressed into a single-layer tablet core with a 9.0 mm round punch.
[0137] Next, wrap the monolayer core with a semipermeable membrane. The film-forming composition comprises 50 wt% cellulose...
Embodiment 2
[0141] A dosage form designed, shaped and adapted to deliver the beneficial drugs levodopa and carbidopa monohydrate to the oral cavity was prepared as follows: First, a drug layer composition was prepared comprising 40.0 wt% LD, 10.8 wt% CD, 31.0Wt% of hydroxypropylcellulose with an average molecular weight of 80,000, 12.7Wt% of mannitol and 5.0Wt% of citric acid, these excipients were sieved through 40 mesh stainless steel, then mixed with 95% ethanol and Granulate until a uniform wet material is formed; the wet material is passed through a 20-mesh stainless steel sieve, and dried at 80° C. for 2 hours; the dried granules are passed through a 18-mesh stainless steel sieve, and then mixed with 0.5 wt % magnesium stearate.
[0142] Next, prepare the second composition, that is, the permeable layer, comprising 55.0Wt% sodium carboxymethylcellulose 7H4XF, 39.0Wt% sorbitol, 5.0Wt% povidone K30 and 0.5Wt% iron oxide red; Stainless steel screen, then mixed with 95% ethanol and gran...
Embodiment 3
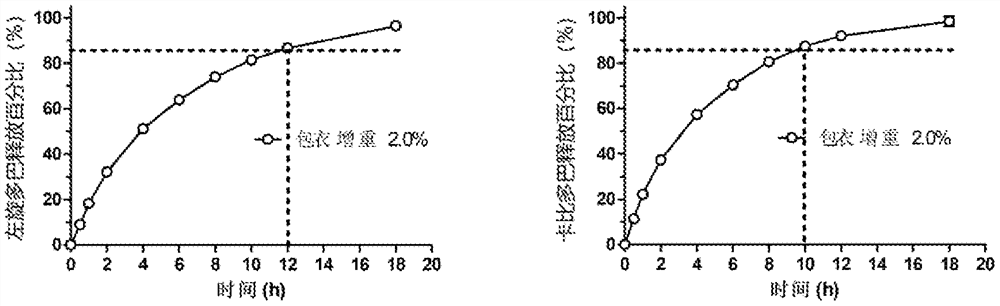
[0146] In this example, the steps of Example 2 are repeated, and the dosage form includes the same drug layer, permeation layer and coating film as those provided in Example 2. In this example, the membrane weight gain was 4.0%, and the delivery orifice size changes were 0.5 mm, 0.75 mm, and 1.0 mm. The final manufactured dosage form delivered LD and CD at average rates of 21.3 mg / hr and 5.7 mg / hr, respectively, delivering 85% of LD / CD within 8.0 hours. Such as Figure 5 As shown, the delivery port size had no significant effect on the release profile. The osmotic delivery system can remain in the oral cavity until the osmotic layer reaches the delivery port, or remain there for 4-5 hours before being swallowed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com