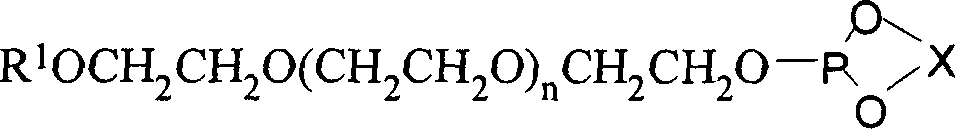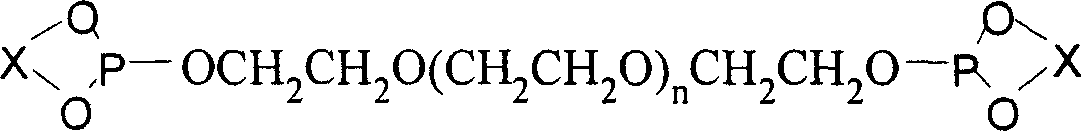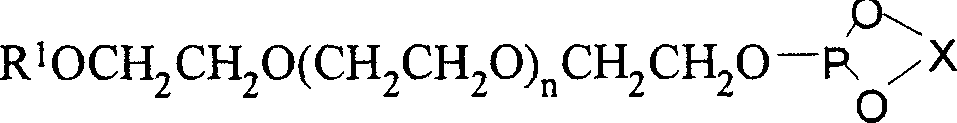Chiral monodentate phosphite ligands, its preparation method and uses
The technology of monodentate phosphite and chlorophosphite is applied in the preparation field of the above-mentioned chiral monodentate phosphite ligand, and achieves the effects of stable properties, simple process route, and stable air and humidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] 1) Synthesis of monodentate phosphite ligand supported by chiral polyethylene glycol
[0078] Add 10 grams of (R)-BINOL and 75 grams of PCl to a 100ml three-necked bottle 3 And a catalytic amount of 2-methylpyrrolidone, the reaction was refluxed until the solid disappeared (about 10 minutes). Remove most of PCl under reduced pressure 3 , a small amount of residual PCl 3 Azeotroped removal with toluene under reduced pressure. After the toluene was removed, the residue was recrystallized from n-hexane to obtain 11.9 g of white phosphorochloride.
[0079] Add 3.51 grams of chlorophosphite and 30 ml of anhydrous dichloromethane into a 100 ml three-necked bottle, slowly add 11 grams of polyethylene glycol monomethyl ether with a molecular weight of 1100 and 3.03 grams of triethylamine to dissolve A solution formed in 20 ml of dichloromethane. After the addition was complete, the reaction solution was raised to room temperature and continued to stir overnight. After fil...
Embodiment 2
[0084] Under nitrogen protection, 2.0mg (0.005mmol) [Rh(COD) 2 ] BF 4 , the chiral polyethylene glycol loaded monodentate phosphite ligand (0.011mmol) prepared above and the solvent methylene chloride (1.5ml) were placed in a 10ml reactor, and after 30 minutes of reaction, the substrate 2 was added - Methyl acetylaminocinnamate (0.5mmol) with 1.5ml CH 2 Cl 2 The formed solution was replaced with hydrogen for 3 times, then reacted under a hydrogen pressure of 10 atm for 12 hours, then terminated the reaction, filtered through a short silica gel column, concentrated the filtered filtrate, and determined its content and optical purity by GC to obtain S-acetylamino The yield of methyl phenylpropionate is 100% (calculated as 2-acetylamino cinnamic acid methyl ester), and the enantiomeric excess is more than 92% ee.
Embodiment 3
[0086] Under nitrogen protection, 2.0mg (0.005mmol) [Rh(COD) 2 ] BF 4 , the monodentate phosphite ligand (0.011mmol) and the solvent methylene chloride (1.5ml) prepared above were placed in a 100ml reactor, reacted for 30 minutes, and added substrate 2 - Methyl acetylaminocinnamate (50mmol) with 25ml CH 2 Cl 2 The formed solution was replaced with hydrogen for 3 times, then reacted under a hydrogen pressure of 10 atm for 12 hours, then terminated the reaction, filtered through a short silica gel column, concentrated the filtered filtrate, and determined its content and optical purity by GC to obtain S-acetylamino The yield of methyl phenylpropionate is 100% (calculated as methyl 2-acetylamino cinnamate), and the enantiomeric excess is more than 90% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com