Pramipexole tablet preparation method, tablet prepared therethrough, and application of tablet
A technology for a cable tablet and a solvent, which is applied in the field of pharmaceutical preparations, can solve the problems of many types of substances and a large total impurity content in the pramipexole tablet, and achieves the effects of a simple and reasonable preparation method, a reduction in the content of related substances, and a low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
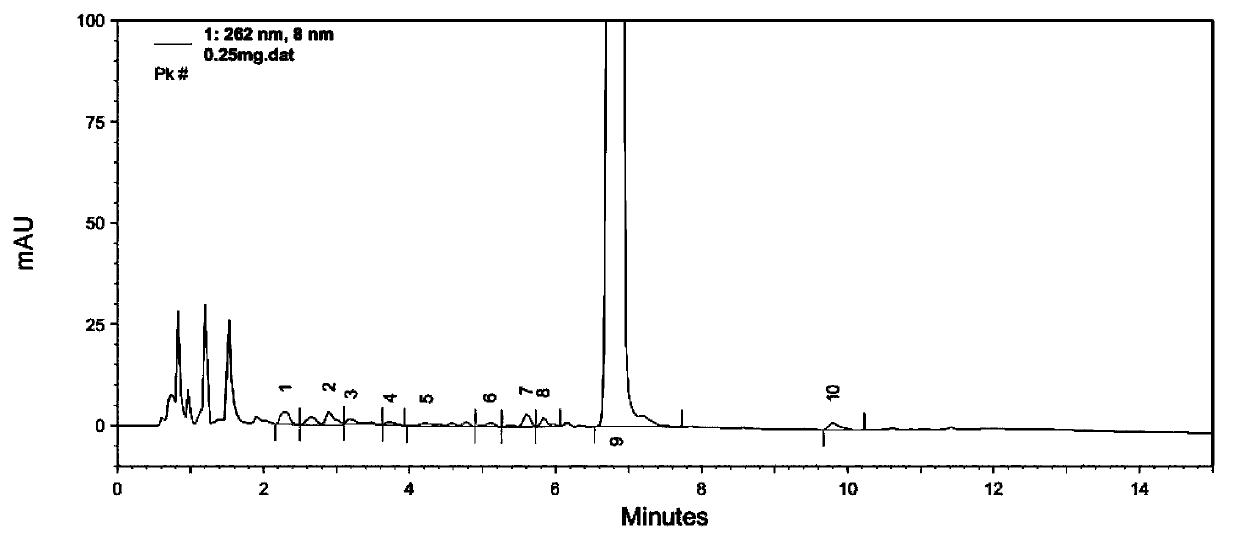
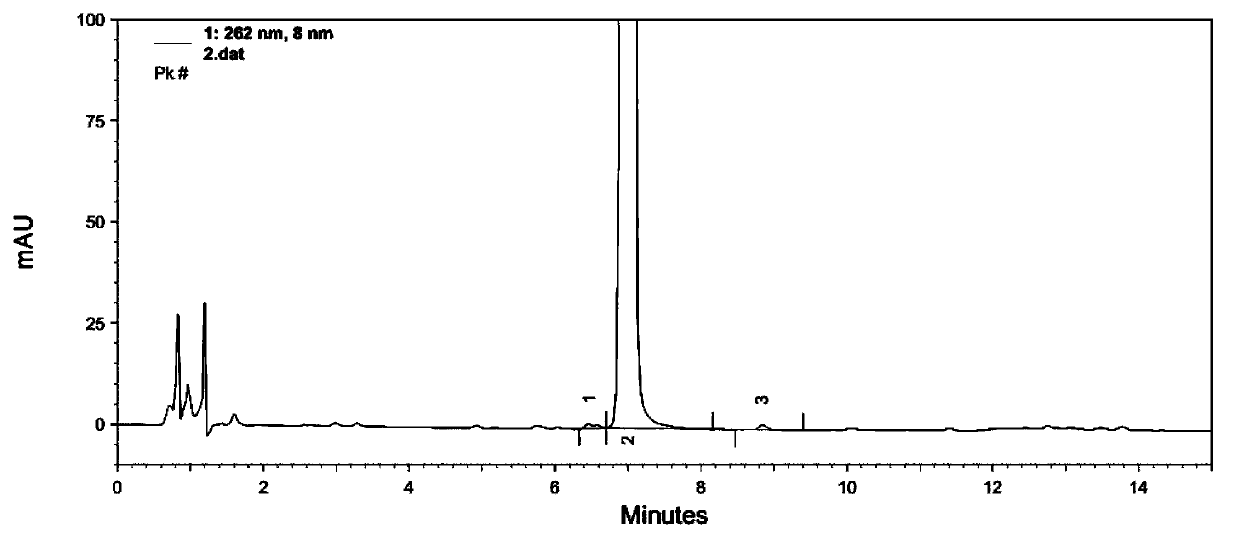
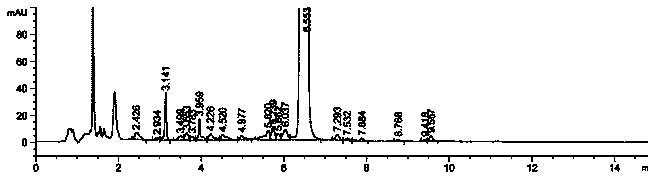
Image
Examples
Embodiment 1
[0049] Example 1 Pramipexole Hydrochloride Tablets (0.125g)
[0050] Prescription, each 1000 tablets contains the following components:
[0051] Pramipexole dihydrochloride monohydrate
125mg
starch
43g
43g
0.45g
silica
0.45g
1.5g
[0052] The preparation method of pramipexole hydrochloride sheet described in the present embodiment, comprises the following steps:
[0053] 1) Suspend 100g of medicinal starch in 100g of 10% (weight) ethanol-water solution, fully swell, disperse, stir for 20min, filter and dry.
[0054] 2) Repeat step 1) 5 times;
[0055] 3) Pass pramipexole hydrochloride, starch, mannitol, and silicon dioxide obtained in step 2) through a 100-mesh sieve, and pass magnesium stearate through a 60-mesh sieve for later use;
[0056] 4) Weigh pramipexole dihydrochloride monohydrate and the starch in step 3) according to the component conten...
Embodiment 3
[0076] Example 3 Pramipexole Hydrochloride Tablets (1g)
[0077] Prescription, each 1000 tablets contains the following components:
[0078] Pramipexole dihydrochloride monohydrate
1g
starch
100g
100g
Magnesium stearate
1.0g
silica
1.0g
6.3g
[0079] 1) Suspend 200g of medicinal starch in 600g of 20% by weight ethanol-water solution, fully swell, disperse, and stir for 20min, then filter and dry;
[0080] 2) Repeat step 1) 5 times;
[0081] 3) Pass pramipexole hydrochloride, starch, mannitol, and silicon dioxide obtained in step 2) through a 100-mesh sieve, and pass magnesium stearate through a 60-mesh sieve for later use;
[0082] 4) Weigh pramipexole dihydrochloride monohydrate and the starch in step 3) according to the component content of 1000 tablets, and fully mix them uniformly by the method of equal increments to obtain a mixed powder;
[0083] 5) Weigh the ma...
Embodiment 4
[0090] Example 4 Pramipexole Hydrochloride Tablets (0.125g)
[0091] Prescription, each 1000 tablets contains the following components:
[0092] Pramipexole dihydrochloride monohydrate
125mg
starch
43g
43g
Magnesium stearate
0.45g
silica
0.45g
4.58g
[0093] 1) Suspend 100g of medicinal starch in 900g of 60% by weight ethanol-water solution, fully swell, disperse, stir for 40min, filter and dry;
[0094] 2) Repeat step 1) once;
[0095] 3) Pass pramipexole hydrochloride, starch, mannitol, and silicon dioxide obtained in step 2) through a 100-mesh sieve, and pass magnesium stearate through a 60-mesh sieve for later use;
[0096] 4) Weigh pramipexole dihydrochloride monohydrate and the starch in step 3) according to the component content of 1000 tablets, and fully mix them uniformly by the method of equal increments to obtain a mixed powder;
[0097] 5) Weigh the mannit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com