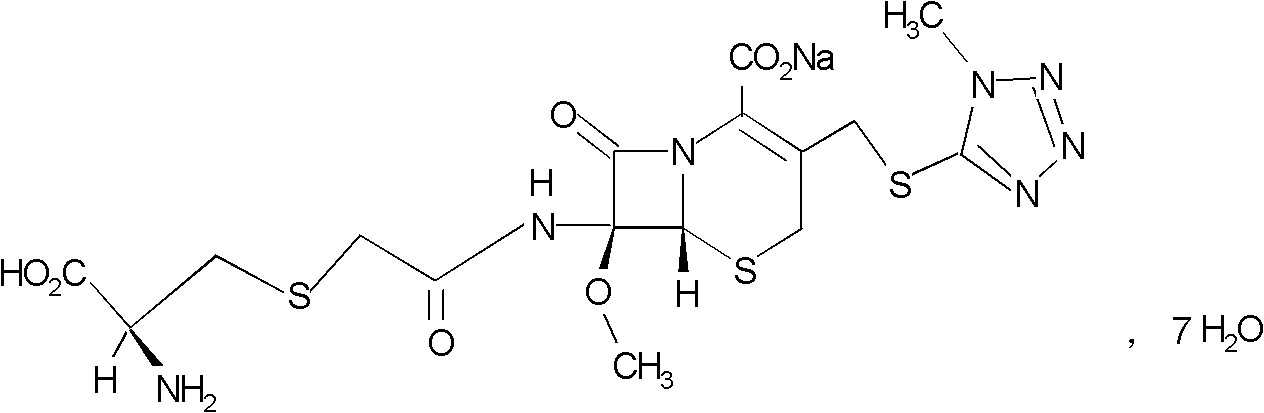
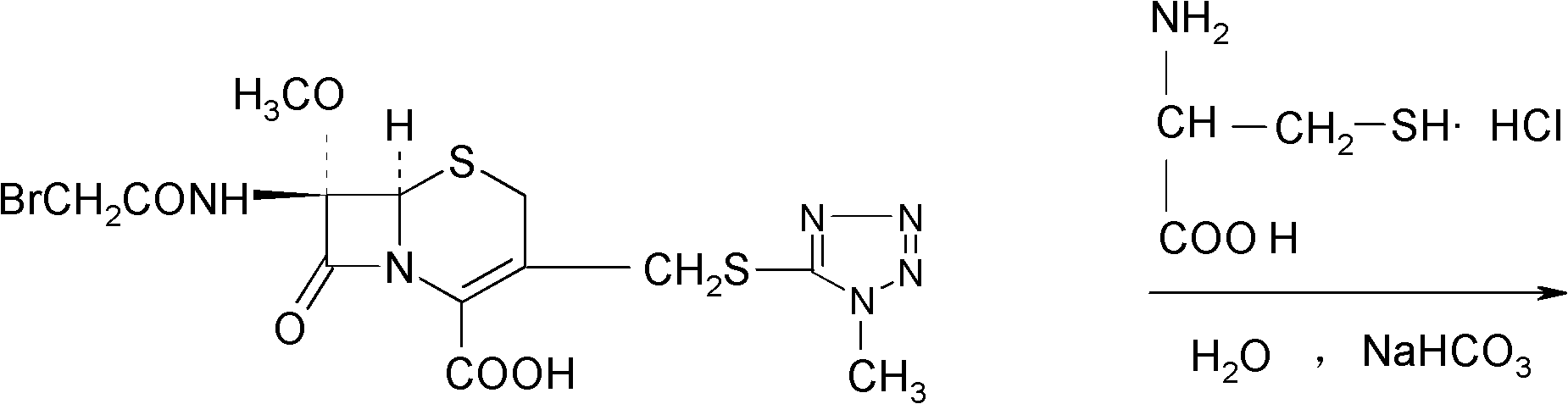
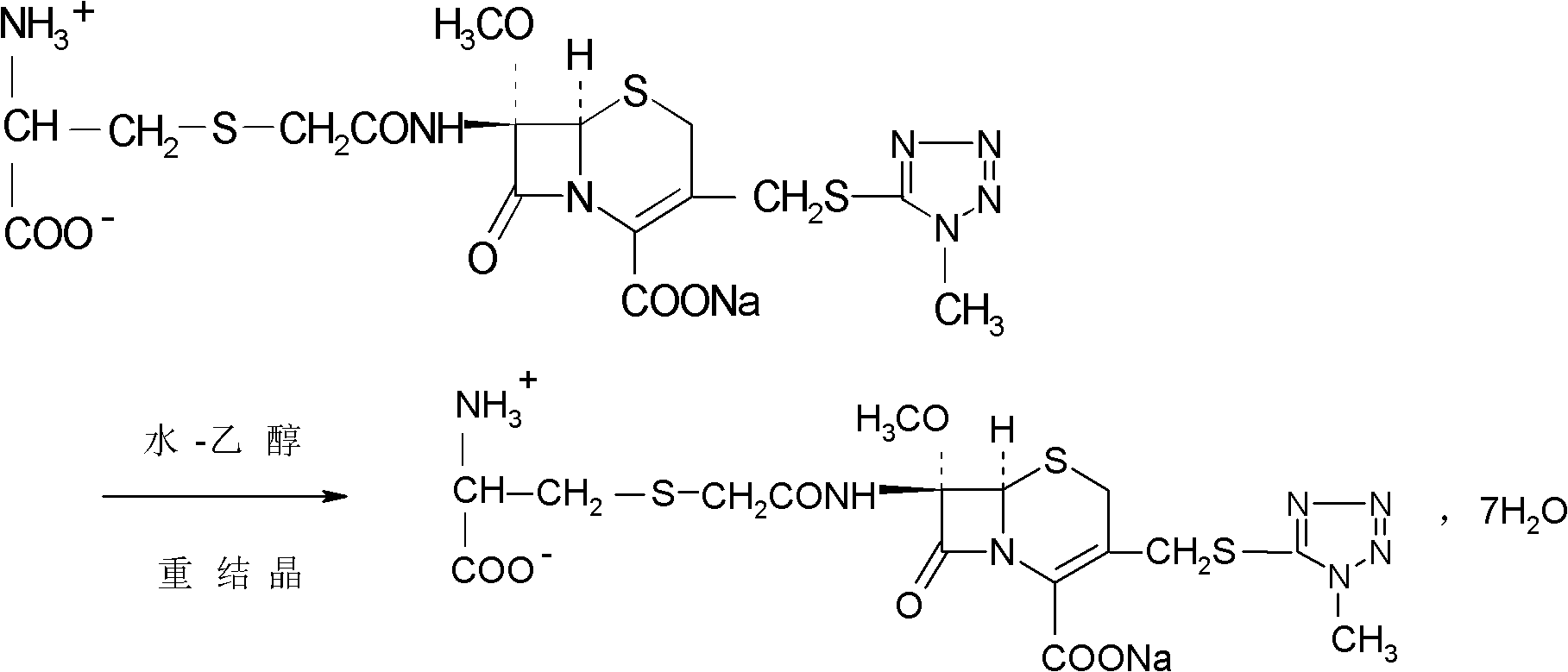
Preparation method of cefminox sodium
A technology for cefminox sodium and cephem, which is applied in the field of preparation of cefminox sodium, can solve the problems of cumbersome processing, unsuitable for industrial application, many purification and purification steps, etc., and achieves simple operation process, uniform crystal form, and fluidity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of cefminox sodium
[0031] 1674.4g (3.52mol) 7β-bromoacetamide-7α-methoxyl group-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid and 855.4 Add g (7.04mol) D-cysteine hydrochloride into 2730ml of water, stir to dissolve, adjust the pH of the reaction system to 6.8 with a 10% aqueous sodium bicarbonate solution by mass percent, and keep stirring at 0°C for 2 hours. Afterwards, the temperature was raised to 2°C, and the reaction was stirred for 2.5 hours, and the stirring rate was maintained at 450 rpm. After the reaction was completed, the reaction liquid was purified by a chromatographic column filled with non-polar macroporous resin X5, and water was used as the eluent. The eluent was collected, concentrated under reduced pressure, cooled, and a solid was precipitated, filtered to obtain 1900 g of a crude solid product, which was dissolved in 7β-bromoacetamide-7α-methoxyl-3-(1-methyl-1H-5-tetrazolyl) Based on thiomethyl-3-cephem-4-carbo...
Embodiment 2
[0035] Preparation of cefminox sodium
[0036]7β-bromoacetamide-7α-methoxy-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid 1674.4g (3.52mol) and D -Add 855.4g (7.04mol) of cysteine hydrochloride into 2730ml of water, stir and dissolve, adjust the pH value of the reaction system to 6.0 with a 10% aqueous solution of sodium bicarbonate, and react with stirring at -5°C After 1.5 hours, raise the temperature to 1.5°C, stir for 1.5 hours, and keep the stirring rate at 300 rpm. After the reaction, the reaction solution is purified by a chromatographic column filled with non-polar macroporous resin X5, and water is used as the elution eluent, collected eluent, concentrated under reduced pressure, cooled, precipitated solid, filtered to obtain 1880g solid crude product, and 7β-bromoacetamide-7α-methoxy-3-(1-methyl-1H-5-tetrazole Based on thiomethyl-3-cephem-4-carboxylic acid, the yield of the crude product is 98.62%, the mp of the crude product is 85.8°C to 86.3°C...
Embodiment 3
[0040] Preparation of cefminox sodium
[0041] 7β-bromoacetamide-7α-methoxy-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid 1674.4g (3.52mol) and D -Add 855.4g (7.04mol) of cysteine hydrochloride into 2730ml of water, stir and dissolve, adjust the pH value of the reaction system to 7.0 with a 10% aqueous solution of sodium bicarbonate with a concentration of 10% by mass, and react with stirring at -3°C After 2.5 hours, raise the temperature to 3°C, stir for 2 hours, and keep the stirring rate at 400 rpm. After the reaction, purify the reaction solution through a chromatographic column filled with non-polar macroporous resin X5, using water as the eluent , collected the eluent, concentrated under reduced pressure, cooled, precipitated solid, and filtered to obtain 1890g solid crude product, which was converted to 7β-bromoacetamide-7α-methoxy-3-(1-methyl-1H-5-tetrazolyl ) thiomethyl-3-cephem-4-carboxylic acid), the yield of the crude product is 99.15%, the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com