A kind of preparation method of linezolid injection
A linezolid and injection technology, applied in the field of pharmaceutical preparations, can solve problems such as unstable content of linezolid and unqualified pyrogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
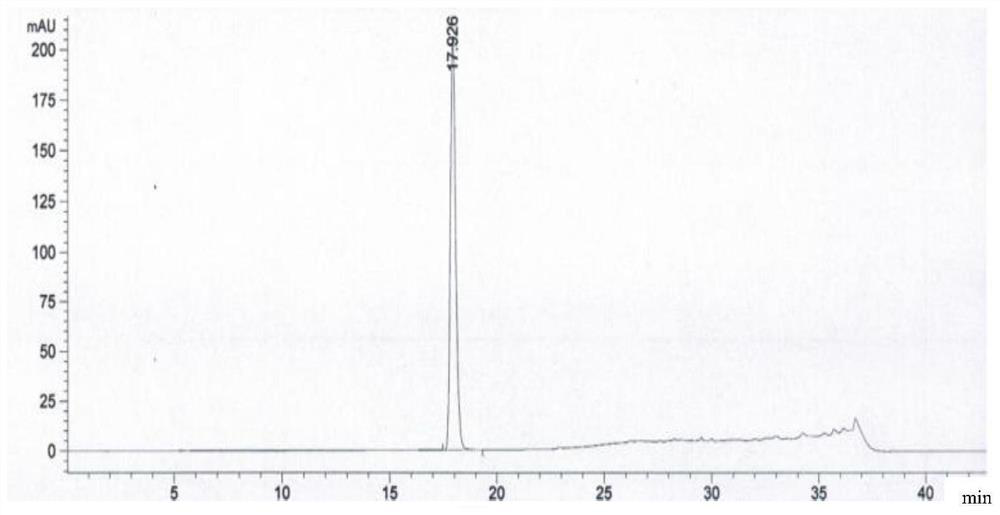
Embodiment 1
[0103] The preparation method of a kind of linezolid injection of the present invention comprises:
[0104] 1) Concentrated
[0105] Under the condition of nitrogen protection, add 400mL of water for injection, sequentially add 2.3g of sodium acetate trihydrate and 1.2g of sodium chloride, place it at 0°C for 18-36 hours, and then add 1.62g of citron Natrate and 1.2g citric acid, stirred until completely dissolved, then added 50g of glucose, stirred until completely dissolved to obtain a medicinal solution, finally added activated carbon with a volume of 0.1% (g / mL) of the medicinal solution to the medicinal solution, and stirred at 60°C After 15 minutes of adsorption, decarburization was carried out, and a 4 μm titanium filter was used to obtain the first filtrate;
[0106] 2) rare match
[0107] Add 2g of linezolid, 0.01g of sodium bisulfite and 0.03g of aluminum chloride to the first filtrate obtained in 1), stir and mix at 80°C, then add 50mL of N-methylpyrrolidone, 10mL...
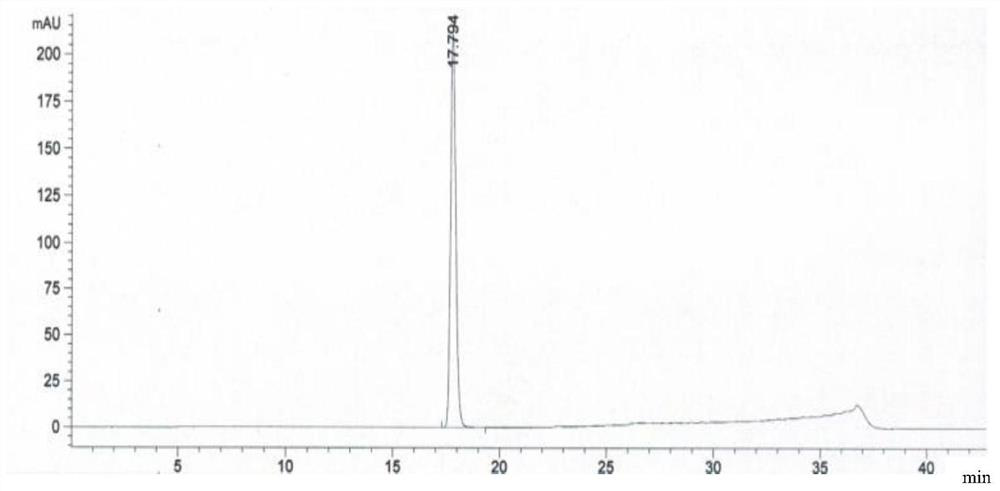
Embodiment 2
[0111] The preparation method of a kind of linezolid injection of the present invention comprises:
[0112] 1) Concentrated
[0113] Under the condition of nitrogen protection, add 400mL of water for injection, sequentially add 2.3g of sodium acetate trihydrate and 1.2g of sodium chloride, place it at 0°C for 18-36 hours, and then add 1.62g of citron Natrate and 0.85g citric acid, stir until completely dissolved, then add 50g glucose, stir until completely dissolved to obtain a medicinal solution, finally add activated carbon with a volume of 0.1% of the medicinal solution (g / mL) to the medicinal solution, and stir at 60°C After 15 minutes of adsorption, decarburization was carried out, and a 5 μm titanium filter was used to obtain the first filtrate;
[0114] 2) rare match
[0115] Add 2g of linezolid, 0.01g of sodium bisulfite and 0.03g of aluminum chloride to the first filtrate obtained in 1), stir and mix at 80°C, then add 50mL of N-methylpyrrolidone, 10mL of dimethyl su...
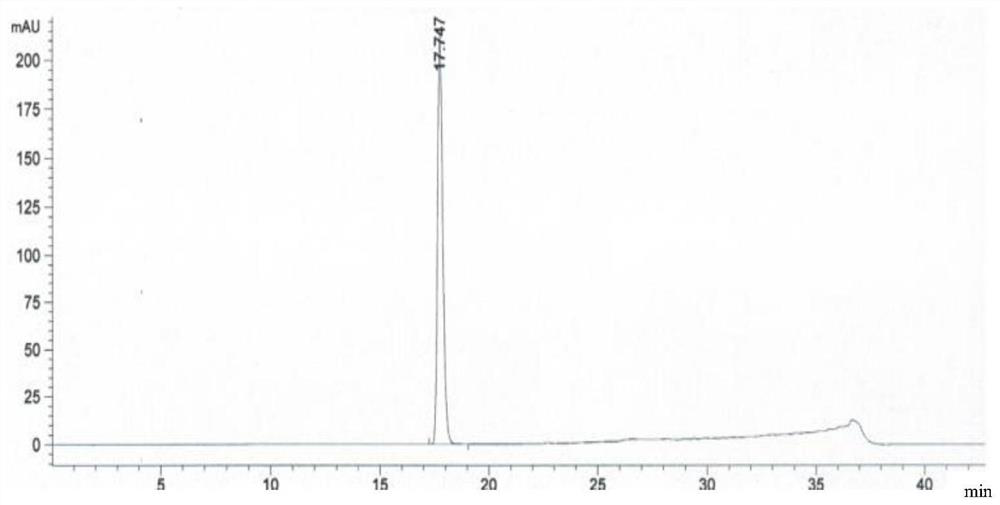
Embodiment 3
[0119] The preparation method of a kind of linezolid injection of the present invention comprises:
[0120] 1) Concentrated
[0121]Under the condition of nitrogen protection, add 400mL of water for injection, sequentially add 2.3g of sodium acetate trihydrate and 1.2g of sodium chloride, place it at 0°C for 18-36 hours, and then add 1.62g of citron Natrate and 1g of citric acid, stirred until completely dissolved, then added 50g of glucose, stirred until completely dissolved to obtain a medicinal solution, and finally added activated carbon with a volume of 0.1% of the medicinal solution (g / mL) to the medicinal solution, and stirred at 60°C for adsorption After 15 minutes, decarburization was performed, using a 6 μm titanium filter to obtain the first filtrate;
[0122] 2) rare match
[0123] Add 2g of linezolid, 0.01g of sodium bisulfite and 0.03g of aluminum chloride to the first filtrate obtained in 1), stir and mix at 80°C, then add 50mL of N-methylpyrrolidone, 10mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com