Preparation method of linezolid injection
A technology of linezolid and injection, applied in the field of pharmaceutical preparations, can solve the problems of unstable content of linezolid, unqualified pyrogen, etc., and achieve the effects of shortening adsorption time and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
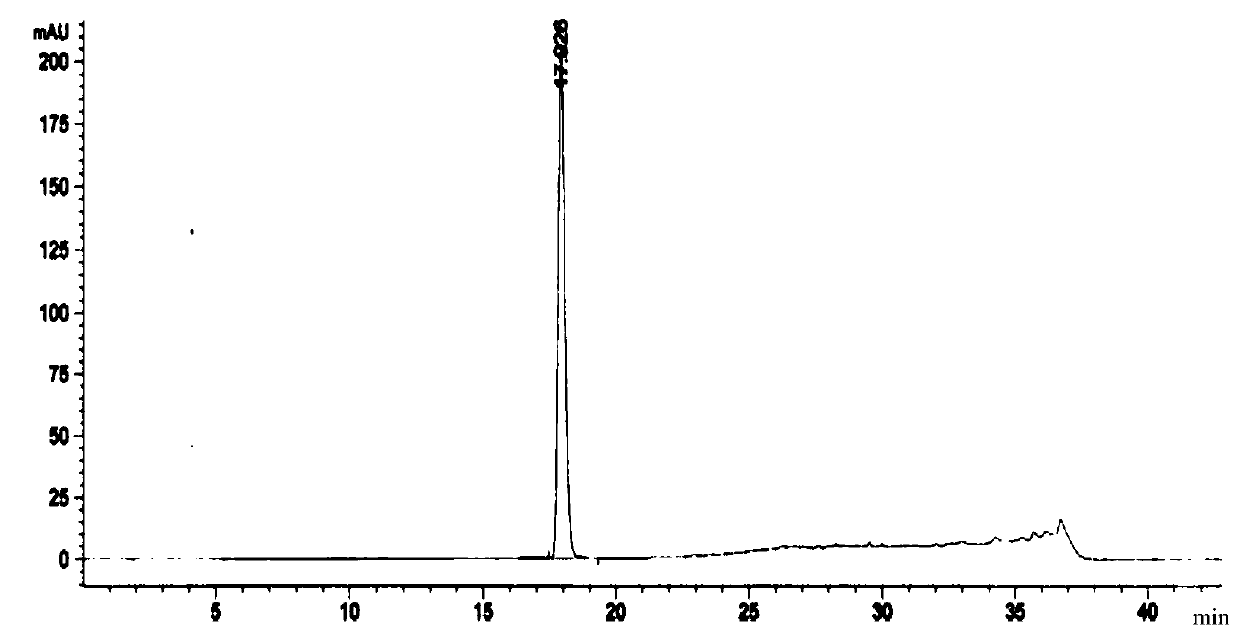
Embodiment 1
[0103] The preparation method of a kind of linezolid injection of the present invention comprises:
[0104] 1) Concentrated
[0105] Under the condition of nitrogen protection, add 400mL of water for injection, sequentially add 2.3g of sodium acetate trihydrate and 1.2g of sodium chloride, place it at 0°C for 18-36 hours, and then add 1.62g of citron Natrate and 1.2g citric acid, stirred until completely dissolved, then added 50g of glucose, stirred until completely dissolved to obtain a medicinal solution, finally added activated carbon with a volume of 0.1% (g / mL) of the medicinal solution to the medicinal solution, and stirred at 60°C After 15 minutes of adsorption, decarburization was carried out, and a 4 μm titanium filter was used to obtain the first filtrate;
[0106] 2) rare match
[0107] Add 2g of linezolid, 0.01g of sodium bisulfite and 0.03g of aluminum chloride to the first filtrate obtained in 1), stir and mix at 80°C, then add 50mL of N-methylpyrrolidone, 10mL...
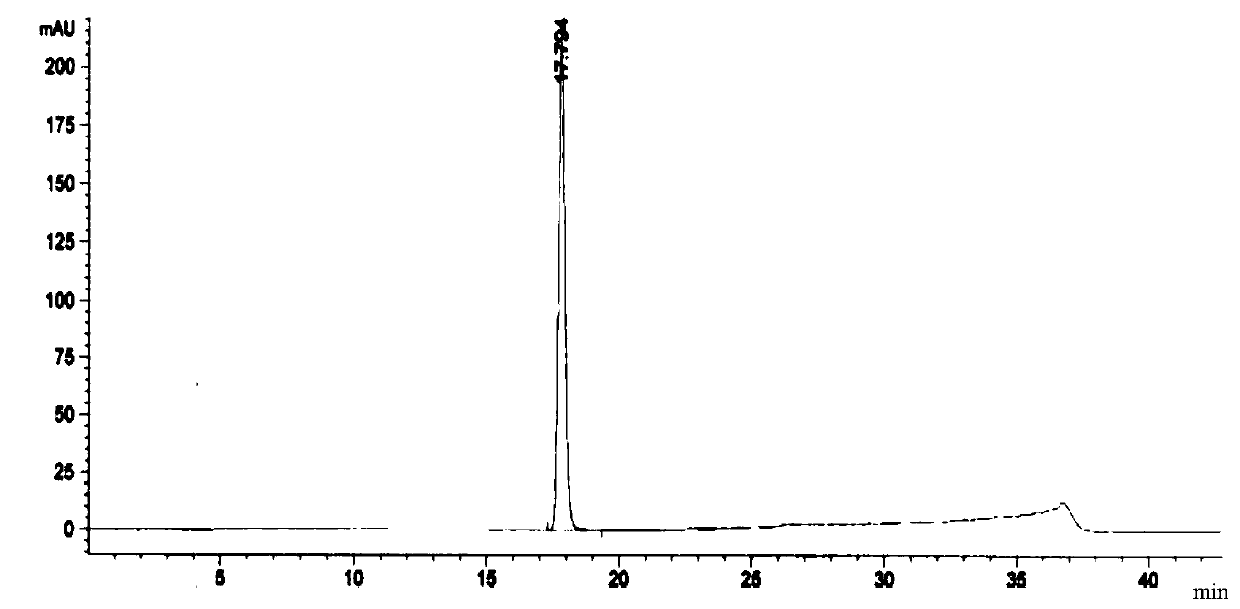
Embodiment 2
[0111] The preparation method of a kind of linezolid injection of the present invention comprises:
[0112] 1) Concentrated
[0113] Under the condition of nitrogen protection, add 400mL of water for injection, sequentially add 2.3g of sodium acetate trihydrate and 1.2g of sodium chloride, place it at 0°C for 18-36 hours, and then add 1.62g of citron Natrate and 0.85g citric acid, stir until completely dissolved, then add 50g glucose, stir until completely dissolved to obtain a medicinal solution, finally add activated carbon with a volume of 0.1% of the medicinal solution (g / mL) to the medicinal solution, and stir at 60°C After 15 minutes of adsorption, decarburization was carried out, and a 5 μm titanium filter was used to obtain the first filtrate;
[0114] 2) rare match
[0115] Add 2g of linezolid, 0.01g of sodium bisulfite and 0.03g of aluminum chloride to the first filtrate obtained in 1), stir and mix at 80°C, then add 50mL of N-methylpyrrolidone, 10mL of dimethyl su...
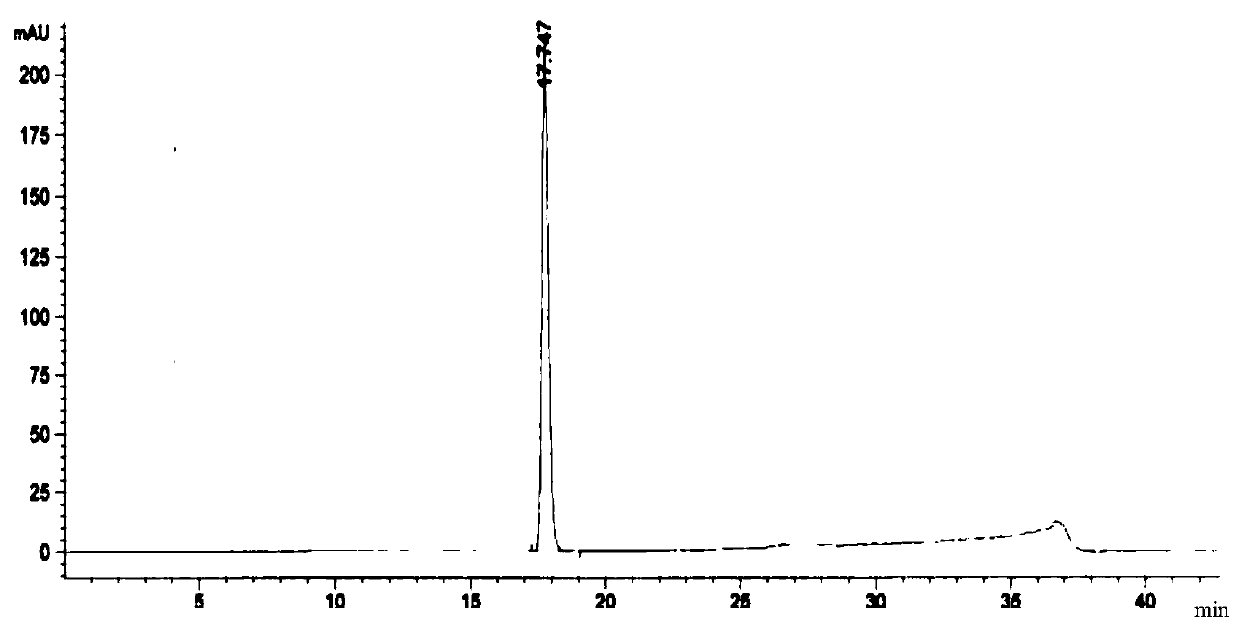
Embodiment 3
[0119] The preparation method of a kind of linezolid injection of the present invention comprises:
[0120] 1) Concentrated
[0121]Under the condition of nitrogen protection, add 400mL of water for injection, sequentially add 2.3g of sodium acetate trihydrate and 1.2g of sodium chloride, place it at 0°C for 18-36 hours, and then add 1.62g of citron Natrate and 1g of citric acid, stirred until completely dissolved, then added 50g of glucose, stirred until completely dissolved to obtain a medicinal solution, and finally added activated carbon with a volume of 0.1% of the medicinal solution (g / mL) to the medicinal solution, and stirred at 60°C for adsorption After 15 minutes, decarburization was performed, using a 6 μm titanium filter to obtain the first filtrate;
[0122] 2) rare match
[0123] Add 2g of linezolid, 0.01g of sodium bisulfite and 0.03g of aluminum chloride to the first filtrate obtained in 1), stir and mix at 80°C, then add 50mL of N-methylpyrrolidone, 10mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com