Medicine composition containing active ingredients of pregabalin
A technology of active ingredients and compositions, applied in the field of pharmaceutical preparations, can solve problems such as unsatisfactory technical effects, and achieve the effects of reducing the number of times of taking medicines, reducing the peak-to-valley ratio, and prolonging the residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 9
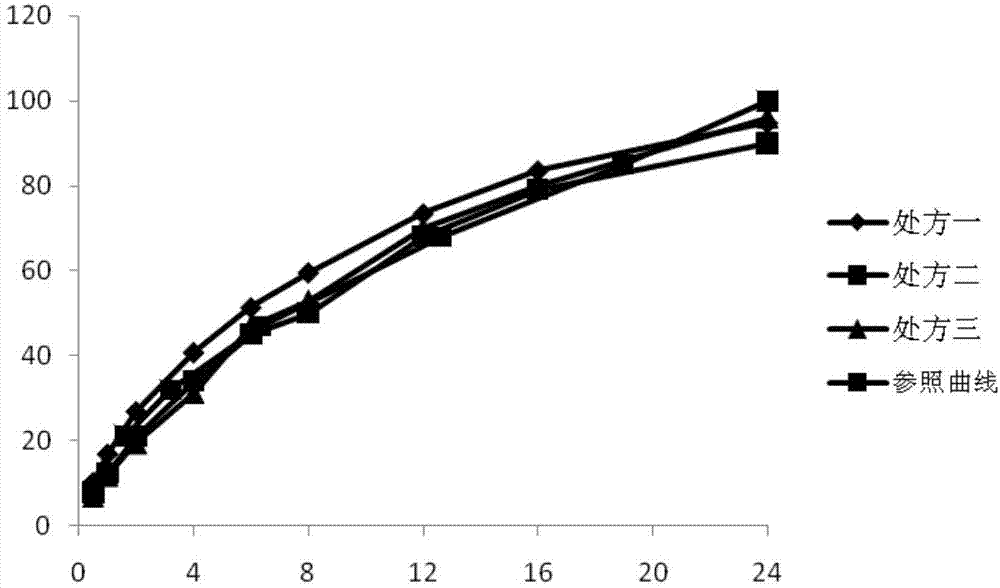
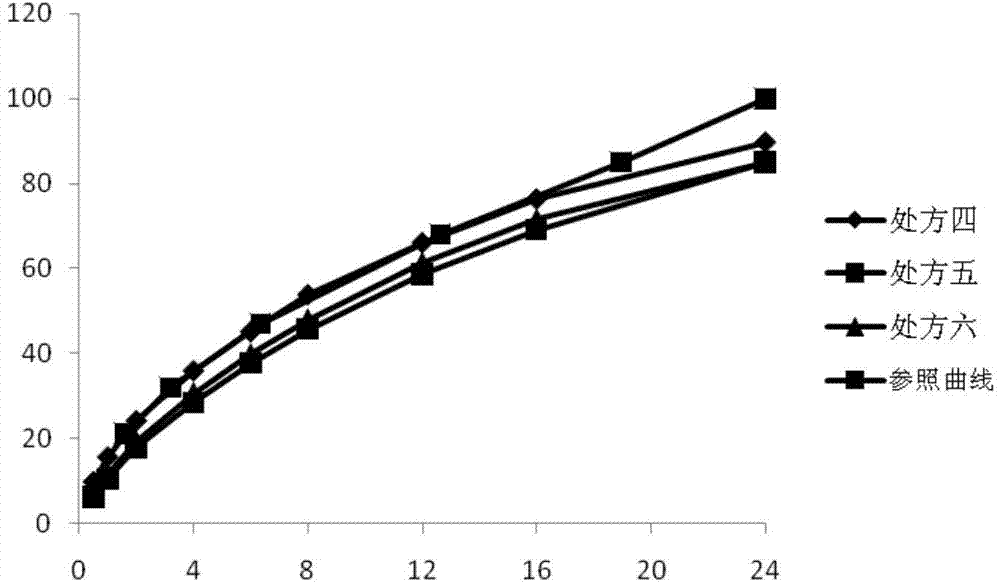
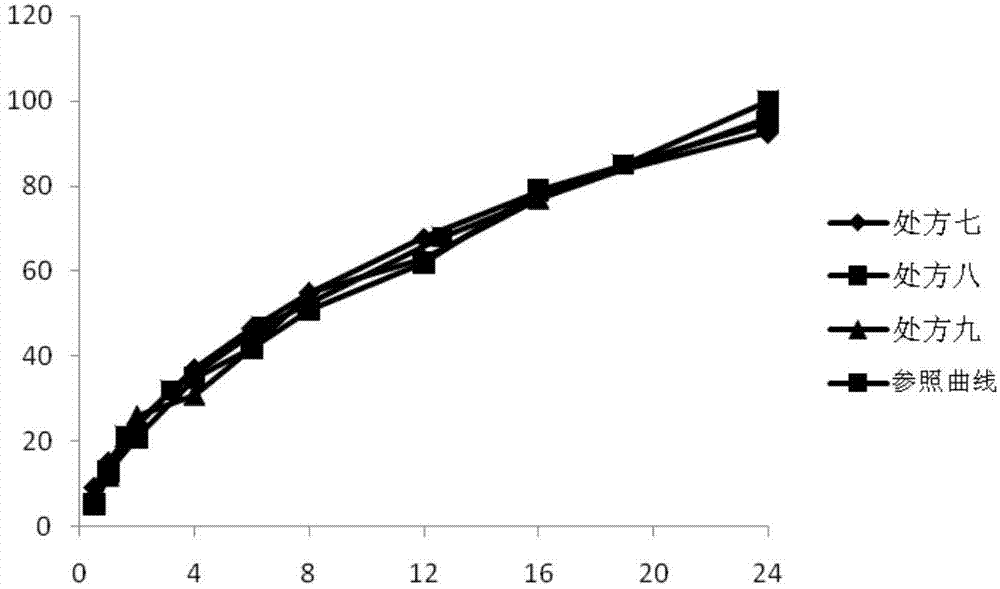
[0028]Prescriptions one to nine in Tables 1, 2, and 3 (corresponding to Examples 1 to 9 respectively) represent laboratory-scale compositions containing pregabalin and various excipients, and Table 4 represents the in vitro swelling of the above compositions and release behavior. Table 5 shows the stability of the examples (percentage of the main impurity lactam). For above embodiment, all components except magnesium stearate are mixed in the hopper mixer, and reach certain mixing homogeneity; Then magnesium stearate is crossed 40 mesh sieves, mix again, at 200 -Compressed into tablets with a tablet weight of 1125mg under a hardness of 300N.
[0029] Table 1 contains the pharmaceutical composition of pregabalin
[0030] composition
Prescription 1 (mg / tablet)
Prescription 2 (mg / tablet)
Prescription Three (mg / tablet)
Pregabalin
75
75
75
Polyvinyl acetate povidone blend
200
345
500
550 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com