Lamivudine tablet composition and preparation method thereof
A technology of lamivudine tablets and lamivudine, which is applied in the field of medicine, can solve problems such as unsatisfactory disintegration effects of preparations, and achieve the effects of good appearance, improved dissolution effect, and delicate appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
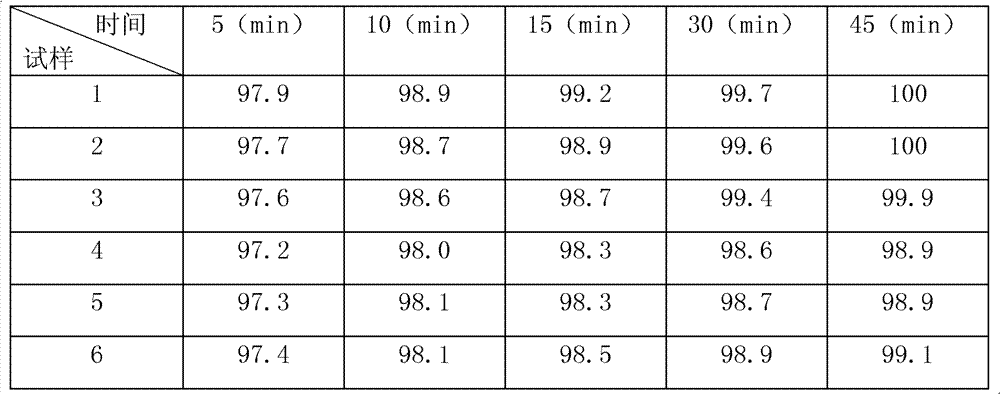
[0061] Get 50% ethanol solution 100ml of lamivudine 100g, microcrystalline cellulose 70g, starch 25g, sodium starch glycolate 18g, magnesium stearate 2g, 5% povidone K30. Get above-mentioned auxiliary material and pass through 80 mesh sieves, get povidone K30, prepare the 50% ethanol solution of 5% povidone K30, standby, take by weighing the microcrystalline cellulose of prescription quantity lamivudine, starch, 10 / 13 and The carboxymethyl starch sodium of 5 / 7, mixes evenly and makes mixed powder, gets 5 / 7 of mixed powder weight, adds 8 / 13 and makes soft material in the 50% ethanol solution of 5% Povidone K30 prepared, makes granules, dried and sized to obtain primary granules with a particle size of about 200-250 mesh. Continue granulating the primary granules, remaining 2 / 7 of the mixed powder and remaining 5 / 13 of the povidone ethanol solution in a fluidized bed until the remaining powder and povidone ethanol solution are completely consumed to obtain intermediate granules,...
Embodiment 2
[0063] Get 50% ethanol solution 100ml of lamivudine 100g, microcrystalline cellulose 70g, starch 25g, sodium starch glycolate 18g, magnesium stearate 2g, 5% povidone K30. Get above-mentioned adjuvant and cross 90 mesh sieves, get povidone K30, prepare 50% ethanol solution of 5% povidone K30, standby, take by weighing the microcrystalline cellulose of prescription quantity lamivudine, starch, 10 / 13 and The carboxymethyl starch sodium of 5 / 7, mixes evenly and makes mixed powder, gets 5 / 7 of mixed powder weight, adds 8 / 13 and makes soft material in the 50% ethanol solution of 5% Povidone K30 prepared, makes granules, dried and sized to obtain primary granules with a particle size of about 200-250 mesh. Continue granulating the primary granules, remaining 2 / 7 of the mixed powder and remaining 5 / 13 of the povidone ethanol solution in a fluidized bed until the remaining powder and povidone ethanol solution are completely consumed to obtain intermediate granules, dried and Whole gra...
Embodiment 3
[0065] Get 50% ethanol solution 100ml of lamivudine 100g, microcrystalline cellulose 70g, starch 25g, sodium starch glycolate 18g, magnesium stearate 2g, 5% povidone K30. Get above-mentioned auxiliary material and pass through 95 mesh sieves, get povidone K30, prepare the 50% ethanol solution of 5% povidone K30, standby, take by weighing the microcrystalline cellulose of prescription quantity lamivudine, starch, 10 / 13 and The carboxymethyl starch sodium of 5 / 7, mixes evenly and makes mixed powder, gets 5 / 7 of mixed powder weight, adds 8 / 13 and makes soft material in the 50% ethanol solution of 5% Povidone K30 prepared, makes granules, dried and sized to obtain primary granules with a particle size of about 200-250 mesh. Continue granulating the primary granules, remaining 2 / 7 of the mixed powder and remaining 5 / 13 of the povidone ethanol solution in a fluidized bed until the remaining powder and povidone ethanol solution are completely consumed to obtain intermediate granules,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com