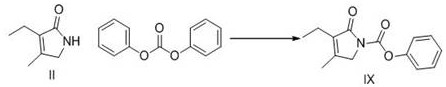
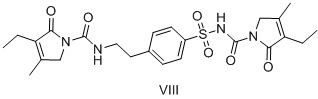
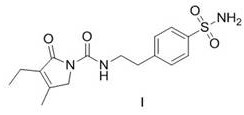
A kind of preparation method of glimepiride intermediate
A technology for intermediates and compounds, applied in the field of API preparation, can solve the problems of impurities Ⅴ and Ⅵ exceeding the standard and exceeding the limit, difficult to remove, and low impurity content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1(1
[0047] Example 1 (1.0 molar equivalent of acetic acid)
[0048] Compound IX. 5.0g, 4-(2-aminoethyl)benzenesulfonamide 4.08g, acetic acid 1.22g and isopropanol 50g heated reflux for 8h (4-(2-aminoethyl)benzene sulfonamide remaining amount of 0.74%), cooled 25 °C, filtered, 50 °C dryed to compound I.6.67g, HPLC detection purity of 99.86%, impurity VIII. 0.05%, impurities V. and VI. undetected, yield 93.2%
Embodiment 2(1
[0049] Example 2 (1.0 molar equivalent acetic acid amplification effect)
[0050] The compound IX. 40.0g, 4-(2-aminoethyl)benzenesulfonamide 32.64g, acetic acid 9.8g and isopropanol 160g were heated and refluxed for 8h, cooled at 25 °C, filtered, and dried at 50 °C to give Compound I. 53.65g, yield 93.6%, HPLC detection purity of 99.78%, impurity VIII. 0.06%, impurity V. and VI. undetected.
Embodiment 3(2
[0051] Example 3 (2.0 molar equivalent of acetic acid)
[0052]The compound IX. 10.0g, 4-(2-aminoethyl)benzenesulfonamide 8.16g, glacial acetic acid 4.9g and isopropanol 50g heated reflux for 7h, cooled 25 °C, filtered, 50 °C dried to compound I. 13.18g, yield 92.0%, HPLC method detection purity of 99.71%, impurities VIII. 0.04%, impurities V. and VI. undetected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com