Polymorph of ALK tyrosine kinase inhibitor and preparation method thereof
A polymorph, isopropyl technology, applied in the polymorph field of pharmaceutical compounds, can solve the problem of drug dissolution rate, different binding force of crystal particles, influence of drug fluidity, particle uniformity, content uniformity and physical stability sexual issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1, N-isopropyl-2-(2-(2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)aniline)-6 , 7-dihydro-5H-pyrrole [2,3-d] pyrimidin-4-ylamine) preparation of benzenesulfonamide polymorph A
[0062] Compound N-isopropyl-2-(2-(2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)aniline)-6,7 -Dihydro-5H-pyrrole[2,3-d]pyrimidin-4-ylamine)benzenesulfonamide (2.0g, 3.15mmol) and N,N-dimethylformamide (10mL) were added to a 50mL three-necked flask heated to 100-105°C, all the solids were dissolved, suction filtered while it was hot, the filtrate was added to a 100mL three-neck flask, heated to 100°C, and isopropanol (30mL) was added dropwise. crystal, filtered, and vacuum dried at 45°C overnight to obtain 1.57 g of a yellow solid, yield: 78.5%.
[0063] HPLC test purity 99.28%.
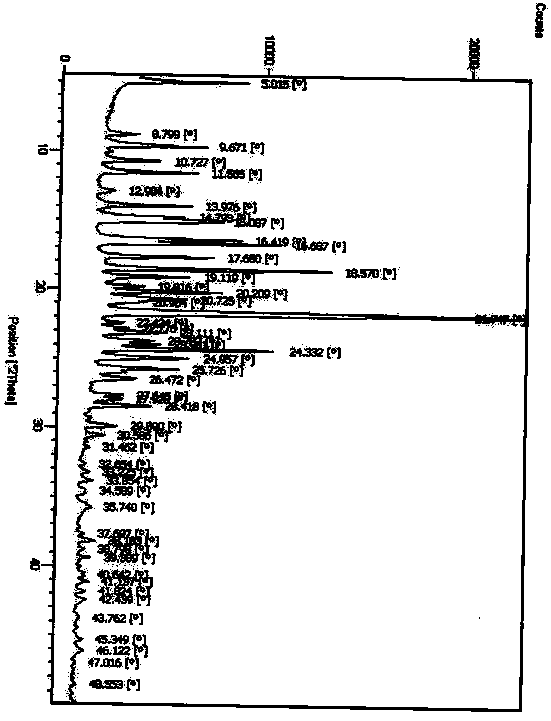
[0064] DSC detection chart (initial temperature 25°C, end temperature 250°C, heating rate 10°C / min) such as Figure 8 shown.
Embodiment 2
[0065] Example 2, N-isopropyl-2-(2-(2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)aniline)-6 , 7-dihydro-5H-pyrrole [2,3-d] pyrimidin-4-ylamine) preparation of benzenesulfonamide polymorph B
[0066] Compound N-isopropyl-2-(2-(2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)aniline)-6,7 -Dihydro-5H-pyrrole[2,3-d]pyrimidin-4-ylamine)benzenesulfonamide (2.0g, 3.15mmol) and trifluoroethanol (10mL) were added in a 50mL three-necked flask, heated to reflux, Dissolve all the solids, filter while hot, add the filtrate to a 100mL three-necked flask, heat to 40-60°C, add ethanol (20mL) dropwise, after the dropwise addition, stir and crystallize at room temperature, filter with suction, and dry under vacuum at 45°C Overnight, 1.72 g of yellow solid was obtained, yield: 86.0%.
[0067] HPLC test purity 99.31%.
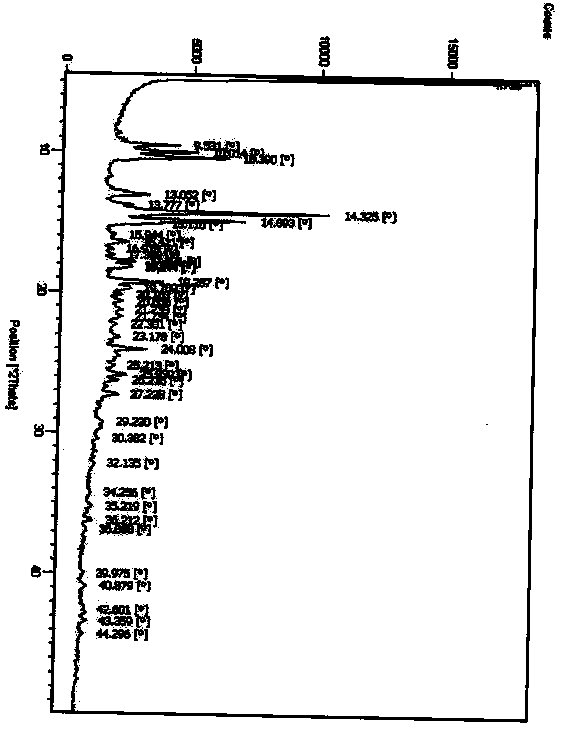
[0068] DSC detection chart (initial temperature 25°C, end temperature 250°C, heating rate 10°C / min)) such as Figure 9 shown.
Embodiment 3
[0069] Example 3, N-isopropyl-2-(2-(2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)aniline)-6 , Preparation of 7-dihydro-5H-pyrrole [2,3-d] pyrimidin-4-ylamine) benzenesulfonamide polymorph C
[0070] Compound N-isopropyl-2-(2-(2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)aniline)-6,7 -Dihydro-5H-pyrrole[2,3-d]pyrimidin-4-ylamine)benzenesulfonamide (2.0g, 3.15mmol) and trifluoroethanol (10mL) were added in a 50mL three-necked flask, heated to reflux, All the solids were dissolved, and suction filtered while it was hot. The filtrate was added to a 100mL three-neck flask, heated to 40-60°C, and isopropanol (20mL) was added dropwise. After the dropwise addition, stirred and crystallized at room temperature, suction filtered, After vacuum drying overnight, 1.75 g of a yellow solid was obtained, yield: 87.5%.
[0071] HPLC test purity 99.23%.
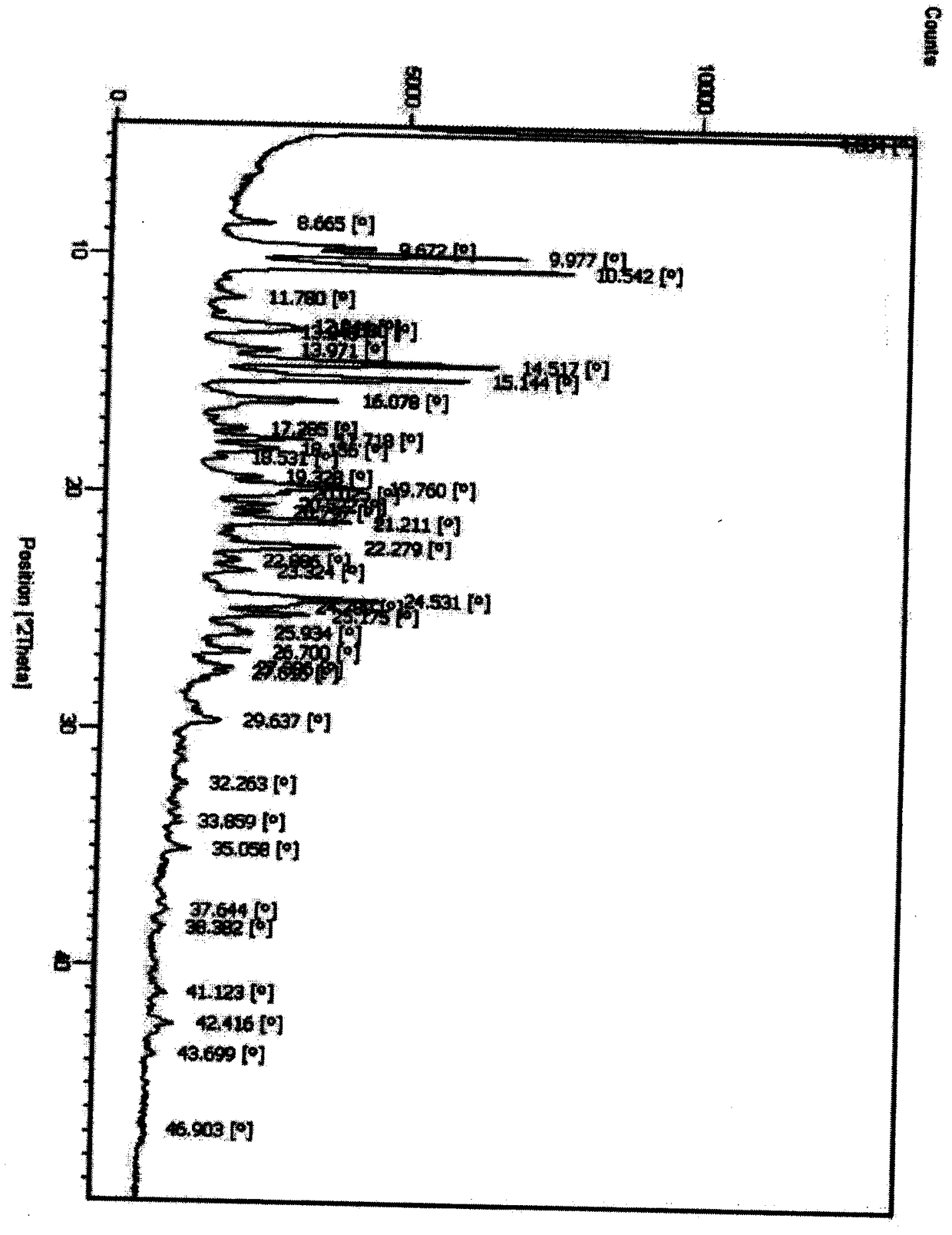
[0072] DSC detection chart (initial temperature 25°C, end temperature 250°C, heating rate 10°C / min) such as Figure 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com