Methods for inhibiting cell proliferation in EGFR-driven cancers
A cancer, subject technology, applied in chemical instruments and methods, botanical equipment and methods, pharmaceutical formulations, etc., to solve problems such as limited resistance to gefitinib and erlotinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1. Kinase Analysis
[0151] In vitro kinase panels were analyzed with WT EGFR, L858R, T790M and L858R / T790M. Additional analyzes can be performed on panels further comprising delE746_A750 and delE746_A750 / T790M. Assay conditions consisted of a 10-point curve with a maximum concentration (single) of 3 μM and 10 μM ATP.
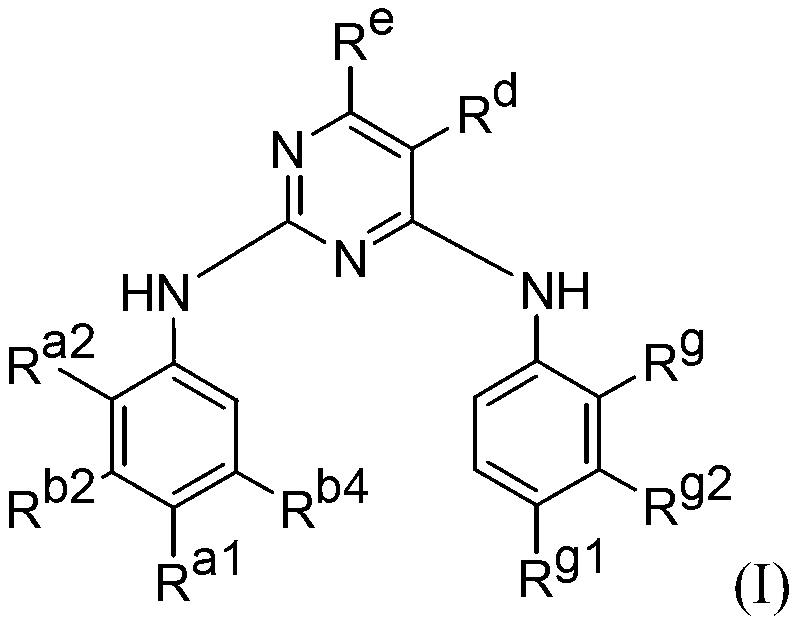
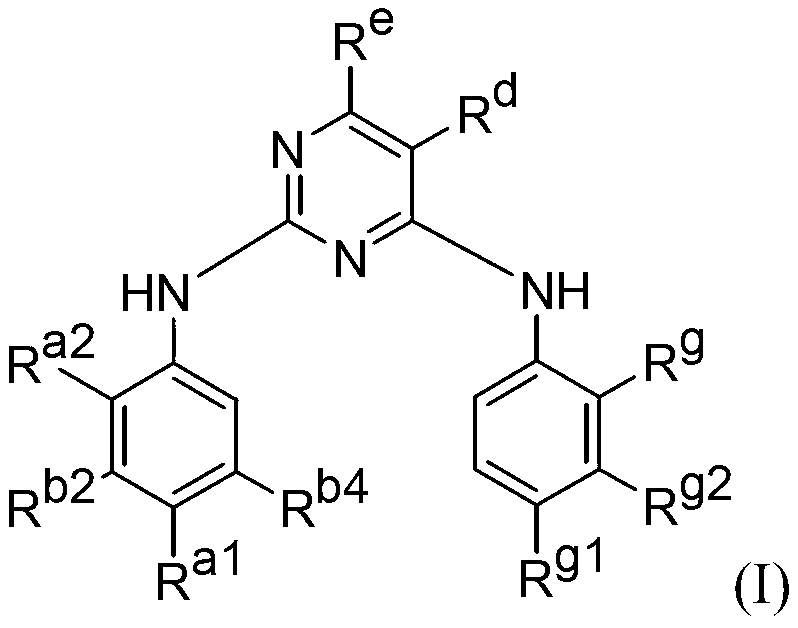
[0152]Compounds of formula (I) include potent inhibitors of EGFR mutants in kinase assays. For example, in the H1975 cell line with the L858R and T790M mutations, previously known inhibitors gefitinib, CL-387,785, and HKI-272 had IC50 values ranging from 153 nM to >3.3 μM, whereas many of the formula (I ) compounds exhibited IC50 values ranging from 0.5 to 9 nM. Therefore, compounds of formula (I) may provide essential inhibitors of EGFR-induced cancers.
Embodiment 2
[0153] Example 2. Cellular and in vivo analysis
[0154] NSCLC cell lines as well as engineered Ba / F3 and NIH3T3 cell lines were used to test the activity of compounds of formula (I) against the following three conventional forms of EGFR: native EGFR (naturally occurring form), EGFR with activating mutations (delE746_A750 [Del] or L858R; this form is sensitive to first-generation EGFR inhibitors) and EGFR with an activating mutation and a T790M resistance mutation (L858R / T790M or Del / T790M; addition of the T790M mutation makes this form sensitive to second-generation EGFR resistance to first-generation EGFR inhibitors). The effect of test compounds on EGFR signaling is assessed by measuring the level of phosphorylated EGFR, the effect on proliferation in vitro is measured by growth and survival assays, and the effect on tumor growth in vivo is measured following daily oral dosing in mice.
[0155] The test compounds of most interest were essentially inactive against native EG...
Embodiment 3
[0159] Example 3. Cell Inhibition Assay
[0160] Lung cancer cell lines were analyzed by measuring the phosphorylation and expression levels of various proteins. For lung cancer cell lines with different EGFR mutant strains, immunoblot analysis of phosphorylation and expression levels of EGFR and other proteins in lung cancer cells was performed. H358 expresses WT EGFR, HCC827 has the delE746_A750 mutation, H820 has the delE746_E749 / T790M mutation, and H1975 has the L858R / T790M mutation.
[0161] Immunoblot analysis was performed on a variety of compounds, including erlotinib, gefitinib, BIBW2992, WZ4003 and several compounds of formula (I).
[0162] This immunoblotting shows that the tested compounds of formula (I) potently inhibit cancer cell lines with EGFR mutations. In particular, the compounds were effective against mutations commonly associated with drug resistance, such as T790M and the combination of L858R and T790M.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com