Application of dihydrofolate synthetase and derivative thereof as well as preparation method and detection method of dihydrofolate synthetase
A technology of dihydrofolate and synthase, which is applied in the biological field to achieve the effect of a rapid detection method and a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Cloning, expression and purification of dihydrofolate synthase
[0064] The primers were designed according to the corresponding gene sequence of EDV67101 published by Genebank. The upstream primer P1 (5'CGCGGATCC ATGAAACTCTTTGC3') and the downstream primer P2 (5'AACTGCAGTTACTCATAGCGTTTG3') contained restriction sites for BamH1 and PstI, respectively. Isolate Escherichia coli F11 from Escherichia coli isolated from chickens with colibacillosis. After culturing, pick a single colony and inoculate it in LB culture medium. Cultivate it until the OD600nm is about 0.4. Take 50 μl of the bacteria solution and centrifuge at a high speed (10000r / min, 1min) , discard the supernatant, add 100 μl PBS to suspend, then centrifuge and suspend again, add 100 μl double distilled water after repeating 3 times, centrifuge and suspend again with double distilled water, then perform PCR to amplify the target gene, and SDS electrophoresis to detect the product.
[0065] Use the D...
Embodiment 3
[0074] Example 3: Using HRP-labeled dihydrofolate synthase to detect sulfonamides in water
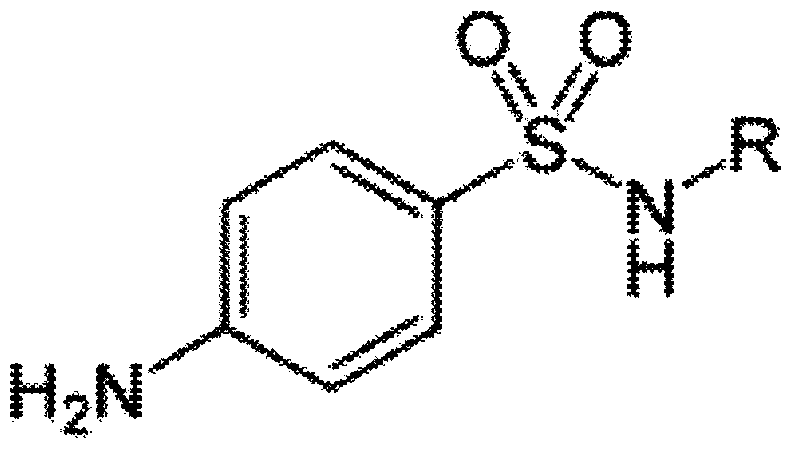
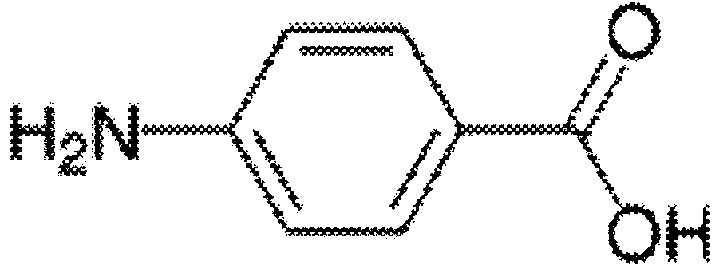
[0075] Coupling p-aminobenzenesulfonamide (V900101, sigma) with ovalbumin OVA by the diazotization method to obtain the original coating SAS-OVA, which was diluted to a concentration of 1 μg / mL with pH 9.6 carbonate buffer as Coating solution was used to coat the microtiter plate with 100 μl per well, and incubated overnight at 4°C. After completion, shake off the liquid, wash the plate with PBS-T, pat dry, add 100 μl of blocking solution (1% skimmed milk powder), incubate at 37°C for 2 hours, shake off the liquid and pat dry for later use.
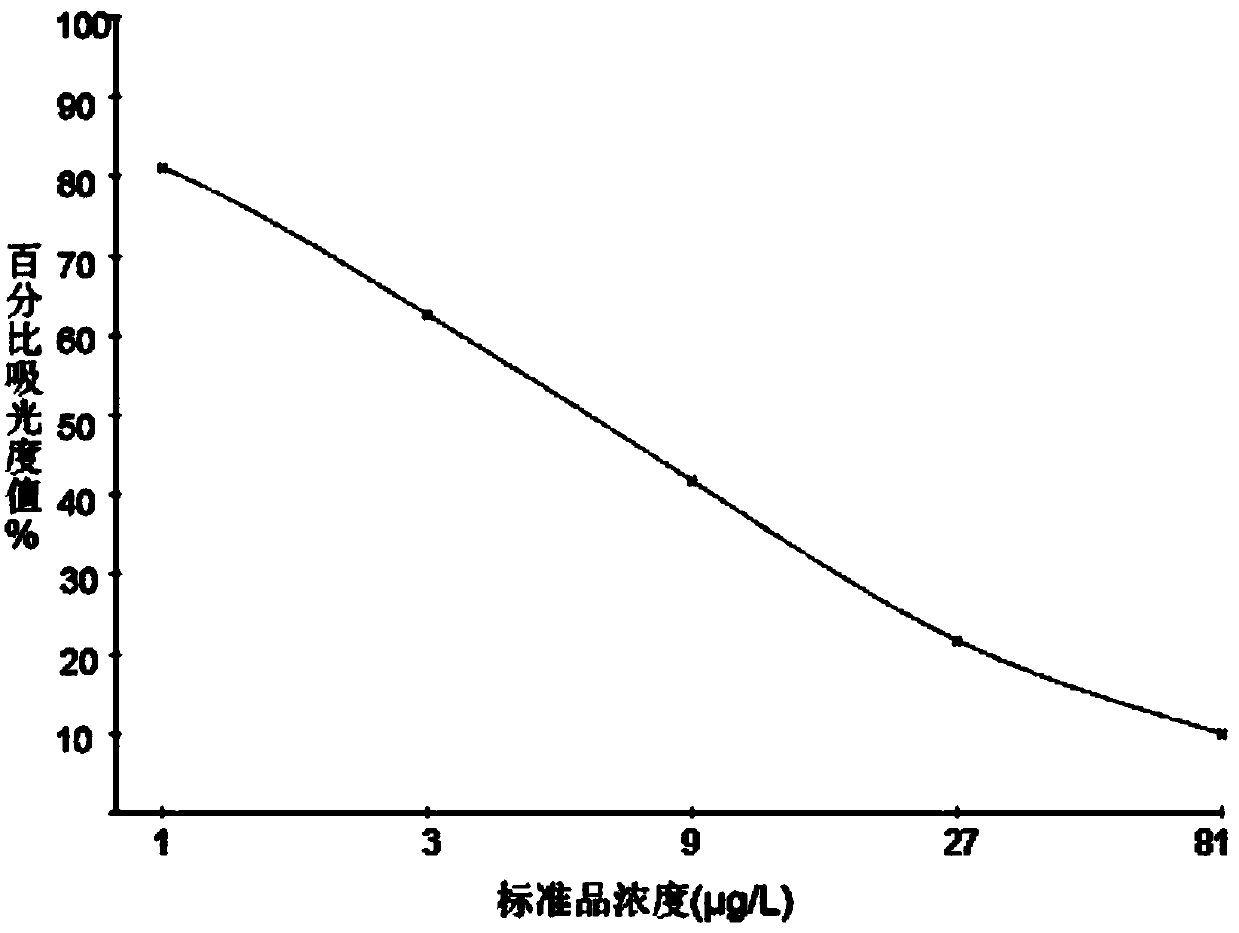
[0076] The concentration of sulfonamide standard preparation is 0μg / L, 1μg / L, 3μg / L, 9μg / L, 27μg / L and 81μg / L.
[0077] Follow the steps below to measure:
[0078] (1) Add standard: Add 50 μl of sulfonamide standard to the coated microtiter plate, and dilute HRP-labeled recombinant dihydrofolate synthase protein to 1 μg / well with 0.1M PBS at pH ...
Embodiment 4
[0082] Example 4: Detection of sulfonamides in milk using colloidal gold-labeled dihydrofolate synthase
[0083] 1. Colloidal gold-labeled dihydrofolate synthase
[0084] The citric acid reduction method is used to prepare a nano-gold particle solution in the range of 20-40 nm, and the pH value is adjusted to 8.0 for later use. Take a certain amount of nano-gold particle solution, place it on a magnetic stirrer, then dilute the purified dihydrofolate synthase with PBS at a ratio of 1:2000, add it to the nano-gold solution and stir for 20-30 minutes, and then put The PEG10000 solution was added to the mixed reaction liquid so that the final concentration of PEG was about 1%, placed in a refrigerated centrifuge (4° C.) and centrifuged at a slow speed (1500-2500 rpm) for 20 minutes, then discarded the supernatant. The lower precipitate was repeatedly washed with PBS and centrifuged to discard excess supernatant. Finally, the precipitate was resuspended in PBS buffer to make the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com